1. Jacobson SH, Eklof O, Lins LE, Wikstad I, Winberg J. Long-term prognosis of post-infectious renal scarring in relation to radiological findings in childhood: a 27-year follow-up. Pediatr Nephrol. 1992; 6:19–24.

2. Montini G, Tullus K, Hewitt I. Febrile urinary tract infections in children. N Engl J Med. 2011; 365:239–50.

3. Ginsburg CM, McCracken GH Jr. Urinary tract infections in young infants. Pediatrics. 1982; 69:409–12.

4. Yilmaz S, Ozcakar ZB, Kurt Sukur ED, Bulum B, Kavaz A, Elhan AH, et al. Vesicoureteral reflux and renal scarring risk in children after the first febrile urinary tract infection. Nephron. 2016; 132:175–80.

5. American Academy of Pediatrics. Committee on Quality Improvement. Subcommittee on Urinary Tract Infection. Practice parameter: the diagnosis, treatment, and evaluation of the initial urinary tract infection in febrile infants and young children. Pediatrics. 1999; 103(4 Pt 1):843–52.
6. Subcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management, Roberts KB. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011; 128:595–610.

7. Oostenbrink R, van der Heijden AJ, Moons KG, Moll HA. Prediction of vesico-ureteric reflux in childhood urinary tract infection: a multivariate approach. Acta Paediatr. 2000; 89:806–10.

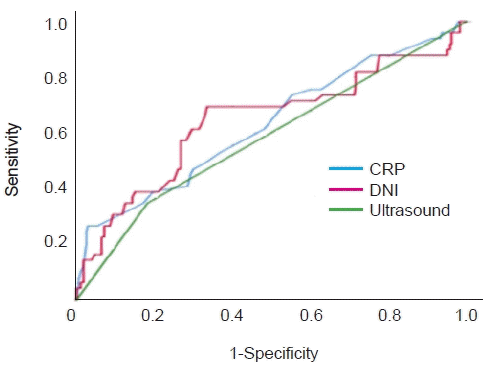
8. Choi EJ, Lee MJ, Park SA, Lee OK. Predictors of high-grade vesicoureteral reflux in children with febrile urinary tract infections. Child Kidney Dis. 2017; 21:136–41.

9. Nahm CH, Choi JW, Lee J. Delta neutrophil index in automated immature granulocyte counts for assessing disease severity of patients with sepsis. Ann Clin Lab Sci. 2008; 38:241–6.
10. Ansari-Lari MA, Kickler TS, Borowitz MJ. Immature granulocyte measurement using the Sysmex XE-2100. Relationship to infection and sepsis. Am J Clin Pathol. 2003; 120:795–9.

11. Mare TA, Treacher DF, Shankar-Hari M, Beale R, Lewis SM, Chambers DJ, et al. The diagnostic and prognostic significance of monitoring blood levels of immature neutrophils in patients with systemic inflammation. Crit Care. 2015; 19:57.

12. Harris N, Kunicka J, Kratz A. The ADVIA 2120 hematology system: flow cytometry-based analysis of blood and body fluids in the routine hematology laboratory. Lab Hematol. 2005; 11:47–61.

13. Lee SH, Ko KO, Lim JW, Yoon JM, Song YH, Lee JW, et al. Delta-neutrophil index: a potential predictor of coronary artery involvement in Kawasaki disease by retrospective analysis. Rheumatol Int. 2019; 39:1955–60.

14. Koh ID, Jeon IS, Kim HM. Diagnostic significance of the delta neutrophil index and other conventional parameters in neonatal bacteremia. Pediatr Infect Vaccine. 2017; 24:1–6.

15. Lee JW, Kim SH, Park SJ, Lee KH, Park JH, Kronbichler A, et al. The value of delta neutrophil index in young infants with febrile urinary tract infection. Sci Rep. 2017; 7:41265.

16. Keren R, Carpenter MA, Hoberman A, Shaikh N, Matoo TK, Chesney RW, et al. Rationale and design issues of the Randomized Intervention for Children With Vesicoureteral Reflux (RIVUR) study. Pediatrics. 2008; 122 Suppl 5(Suppl 5):S240–50.

17. Montini G, Rigon L, Zucchetta P, Fregonese F, Toffolo A, Gobber D, et al. Prophylaxis after first febrile urinary tract infection in children? A multicenter, randomized, controlled, noninferiority trial. Pediatrics. 2008; 122:1064–71.

18. Pennesi M, Travan L, Peratoner L, Bordugo A, Cattaneo A, Ronfani L, et al. Is antibiotic prophylaxis in children with vesicoureteral reflux effective in preventing pyelonephritis and renal scars? A randomized, controlled trial. Pediatrics. 2008; 121:e1489–94.

19. Hodson EM, Wheeler DM, Vimalchandra D, Smith GH, Craig JC. Interventions for primary vesicoureteric reflux. Cochrane Database Syst Rev. 2007; (3):CD001532.

20. Koyle MA, Elder JS, Skoog SJ, Mattoo TK, Pohl HG, Reddy PP, et al. Febrile urinary tract infection, vesicoureteral reflux, and renal scarring: current controversies in approach to evaluation. Pediatr Surg Int. 2011; 27:337–46.

21. Lim R. Vesicoureteral reflux and urinary tract infection: evolving practices and current controversies in pediatric imaging. AJR Am J Roentgenol. 2009; 192:1197–208.

22. Merguerian PA, Sverrisson EF, Herz DB, McQuiston LT. Urinary tract infections in children: recommendations for antibiotic prophylaxis and evaluation: an evidence-based approach. Curr Urol Rep. 2010; 11:98–108.

23. Riccabona M, Avni FE, Blickman JG, Dacher JN, Darge K, Lobo ML, et al. Imaging recommendations in paediatric uroradiology: minutes of the ESPR workgroup session on urinary tract infection, fetal hydronephrosis, urinary tract ultrasonography and voiding cystourethrography, Barcelona, Spain, June 2007. Pediatr Radiol. 2008; 38:138–45.

24. Williams GJ, Hodson EH, Isaacs D, Craig JC. Diagnosis and management of urinary tract infection in children. J Paediatr Child Health. 2012; 48:296–301.

25. National Collaborating Centre for Women's and Children's Health. Urinary tract infection in children: diagnosis, treatment and long-term management. London: RCOG Press;2007.
26. Park BH, Kang YA, Park MS, Jung WJ, Lee SH, Lee SK, et al. Delta neutrophil index as an early marker of disease severity in critically ill patients with sepsis. BMC Infect Dis. 2011; 11:299.

27. Eskandarifar A, Rahehag R, Jafari M. Evaluating the measurement of urinary neutrophil gelatinase associated lipocalin for the diagnosis of vesicoureteral reflux in children. Int J Pediatr. 2021; 9:15015–21.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download