Abstract
Purpose
To determine predictive factors for detecting renal parenchymal damages (RPDs) in infants with recurrent febrile urinary tract infection (fUTI).
Methods
From January 2015 to December 2021, 102 infants with recurrent fUTI and who underwent 99mTc-dimercaptosuccinic acid (DMSA) renal scan in our hospital were included in this study. Controls included infants with normal DMSA results performed 3 months apart from the 2nd episode of fUTI. DMSA-positive group included infants with positive DMSA results performed 3 months apart from the 2nd episode of fUTI or at the 3rd episode of fUTI. The recurrence rate, causative bacteria, renal size discrepancy of both kidneys, and laboratory findings including C-reactive protein (CRP) and spot urine sodium-to-potassium ratio (uNa/K) were compared between both groups.
Results
Only 3.8% of 79 infants with a 2nd episode of fUTI showed positive DMSA results. fUTI recurred more frequently within 12 months of follow-up in the DMSA-positive group than in the control group (69% vs. 13%, P<0.001). CRP values were significantly higher in the DMSA-positive group than in the control group (7.3 mg/dL vs. 3.7 mg/dL, P<0.001). Spot uNa/K were significantly lower in the DMSA-positive group than in the control group (0.6 vs. 1.1, P<0.001).
Febrile urinary tract infection (fUTI) is common in infants who are immunocompromised and lack a defense mechanism against bacterial ascending infection [1]. The fUTI imaging strategies used worldwide include a top-down approach (99mTc-dimercaptosuccinic acid [DMSA] renal scan is the first imaging study) and a bottom-up approach (voiding cystourethrography [VCUG] is the imaging study prior to DMSA scan) [2].
Currently, routine DMSA renal scan and VCUG are not recommended during the 1st episode of fUTI because of radiation exposure [3]. However, despite differing opinions, renal ultrasonography (US) may be beneficial during the 1st episode of fUTI in order to exclude renal abscess or other accompanying congenital anomalies of the kidney and urinary tract (CAKUT) if no economic hindrance exists [4].
Therefore, a less invasive and less expensive urinary tract imaging strategy that can detect high-risk patients possessing lower glomeruli number than normal whose urinary tract infection (UTI) is susceptible to progress into chronic renal injury (CRI) during their lifetime would be ideal. Furthermore, the fewer the number of practiced imaging studies, the more ideal that strategy would be.
Congenital hypoplastic kidney or congenital renal scar (cRS) is likely to progress into CRI due to recurrent pyelonephritis and thus should be diagnosed as early as possible [5]. Fortunately, the incidence of simple renal hypoplasia is very low (0.27%) compared with that of fUTI [6]. Most patients with cRS also have high-grade vesicoureteral reflux (VUR) [7].
High-grade VUR, a common anomaly of CAKUT causing recurrent fUTI, is a main target of the bottom-up approach in the fUTI imaging strategy mainly because it is an anomaly that needs early surgical management in children with fUTI who have renal scars [8]. Nonetheless, if further fUTI does not recur, an acquired renal scar (aRS) is less likely to develop in the future. Among children with fUTI, pyelitis that does not cause renal scarring is more common than pyelonephritis that causes renal scarring [9]. If renal scarring does not occur in children with fUTI, immediate surgical treatment is not needed, and if eventually needed, it could be postponed for a later time. Hence, we prefer performing a DMSA scan before a VCUG during the 2nd episode of fUTI.
Renal scarring can lead to renal complications like proteinuria or hypertension, and progress into CRI through recurrent pyelonephritis and so on [10].
There have been many reports studying the factors predicting renal scarring in fUTI children. Procalcitonin, high-grade VUR, previous renal scarring, urine pentraxin-3, plasma neutrophil gelatinase-associated lipocalin, and urinary interleukin-6 or interleukin-8 positively correlated with the presence of renal scarring in fUTI children [11-15]. Although procalcitonin was a better predictor of renal scarring, it was more expensive than C-reactive protein (CRP). The other predicting factors were clinically impractical methods in the general clinical field and correlated with the presence of VUR.
Some authors have published a few reports pertaining to spot urine sodium-to-potassium ratio (uNa/K) as a useful predictor of acute pyelonephritis in fUTI children excluding pyelitis or lower UTI with other fever focus [16-18]. In these studies, authors paid attention whether spot uNa/K could be useful for predicting renal parenchymal damages (RPDs) leading into renal scar.
The aim of this study was to retrospectively analyze the laboratory and radiological findings in children with fUTI who had undergone a DMSA scan in our hospital according to our urinary tract imaging strategy and to determine the factors that predict the presence of RPDs including spot uNa/K.
This study included a total of 134 children who had been admitted to our hospital with a past history of more than two episodes of fUTI and had performed a DMSA scan in our hospital between January 2015 and December 2021. Of the 134 children, 32 were excluded: five patients underwent DMSA scans during the acute phase of infection during their 2nd episode of fUTI, nine patients had accompanying CAKUT, except hydronephrosis on US, and 18 patients had no CRP, urine Na, urine K data. Finally, this study enrolled 102 children, with 26 patients in the DMSA-positive group and 76 patients in the control group.
fUTI was defined as high fever (≥38°C), pyuria (>5 white blood cells [WBCs]/high-power field), positive leukocyte esterase on urinalysis, positive serum CRP values, and no other fever focus at admission.
Our urinary tract imaging strategy was as follows: for the 1st episode of fUTI, an US was performed. Then, a DMSA scan was performed 3 months after the acute infection in cases with either a suspicion of hypoplastic kidney (small kidney, less than 3 percentile of normal range) on the US during the 1st episode or in cases with a 2nd episode of fUTI (Fig. 1). In cases where fUTI recurred within 3 months from the 2nd episode, a DMSA scan was performed during the acute phase of infection at the 3rd episode (Fig. 1). VCUG was recommended in patients whose DMSA scan revealed positive findings. Significant bacteriuria was defined as 100,000 colony-forming units/mL of a single strain isolate. Urine samples were collected with a clean catch bag. At admission, blood samples (CRP, WBC, etc.) and spot urine samples (urinalysis, urine Na, urine K, etc.) were collected. All blood and urine samples from the 1st and 2nd episodes were included in the study. Urine electrolytes sampled over 24 hours after the start of intravenous hydration were excluded because those values change to a great extent with increased intravascular volume. CRP was measured by turbidimetry. DMSA scans were performed with planar technique and were interpreted by both a nuclear medicine consultant and a pediatric nephrologist.
Patients whose DMSA scan showed a small kidney (less than 3 percentile of normal range) with either diffuse cortical defects with deformative renal contour or with a relative renal uptake of less than 35% in a kidney were classified as having cRS and were included in the DMSA-positive group. Mild cortical defects were defined as the presence of one or two cortical defects (photopenia) with normal renal contour line. Moderate to severe cortical defects were defined as the presence of multiple focal cortical defects (≥3) on the DMSA scan. Patients with normal DMSA findings were included in the control group. We compared clinical characteristics, CRP, WBC, uNa/K, urine culture, and renal US between the DMSA-positive and control groups. Mean values of blood and urine samples from the 1st and 2nd episodes in each patient were calculated. All data were retrospectively analyzed.
All variables are presented as mean±standard deviation, and Student t-test was used when factors were compared between two independent groups. Continuous variables were analyzed using Wilcoxon-Mann-Whitney test. Statistical significance was defined as P≤0.05.
Among 79 infants with the 2nd episode of fUTI, only three (3.8%) showed positive DMSA results on the scan performed 3 months after the last episode, including an infant who had cRS. Among 27 infants with a 3rd episode of fUTI, four (14.8%) had normal renal cortices, eight (29.6%) had mild cortical defects, eight (29.6%) had cRS, and seven (25.9%) had moderate to severe cortical defects without cRS (with a possibility of aRS developing in the future) on the acute DMSA scan. Four infants with a 3rd episode of fUTI were excluded from the DMSA-positive group because of missing data.
The DMSA-positive group included three infants who underwent DMSA scanning 3 months after the 2nd episode of fUTI and 23 infants who underwent acute DMSA scanning during the 3rd episode of fUTI.
fUTI recurred more frequently in the DMSA-positive group than in the control group within 12 months of follow-up after their DMSA scans were performed (34.6% vs. 13.2%, P=0.01) (Table 1). The DMSA-positive group had a male predominance compared with the control group (92.3% vs. 72.4%, P=0.03).
The mean interval period between the 1st and 2nd episodes of fUTI, culture-negative pyelonephritis, non-Escherichia coli bacteria (bacteria causing fUTI except E. coli), and the presence of extended-spectrum beta-lactamase-producing E. coli as causative bacteria were not significant between the DMSA-positive and control groups (Table 1).
Serum CRP values (normal range ≤0.03 mg/dL) were significantly higher in the DMSA-positive group than in the control group (7.3 mg/dL vs. 3.7 mg/dL, P<0.001). The differences in serum WBC and procalcitonin values were not significant between the two groups (P=0.12 and P=0.09, respectively). However, procalcitonin was not sampled in some of the patients in this study.
Spot uNa/K in urine samples collected within 24 hours after admission was significantly lower in the DMSA-positive group than in the control group (0.6 vs. 1.1, P<0.001).
Renal US revealed that only three infants with cRS had a significantly large size discrepancy in both kidneys (1.9, 1.9, and 2.7 cm), whereas the others with cRS had no significant difference in the size discrepancy of both kidneys compared with the control group (P=0.24).
VCUG was recommended to all infants enrolled in the DMSA-positive group. However, the parents of 13 infants at our hospital objected to VCUG, including two infants with cRS, four infants with moderate to severe cortical defects, and seven infants with mild cortical defects on the DMSA scan. Thirteen infants in the DMSA-positive group underwent VCUG in this study. All enrolled infants had VUR, with one, three, four, and five infants having VUR grades 2, 3, 4, and 5, respectively. Bilateral VUR was detected in four infants (31%) (Table 2). Seven infants with cRS, with one, one, three, and two infants having VUR grades 2, 3, 4, and 5, respectively. Nine infants with VUR grades 4 and 5 included five infants with cRS, one infant with mild cortical defects, and three infants with moderate to severe cortical defects (Table 2).
The recurrence of fUTI has already been known as a strong predisposing factor of aRS regardless of age of occurrence [19]. This study also showed that infants with positive DMSA results had more frequent episodes of fUTI than controls; however, the follow-up period was short. In this study, only 3.8% of infants with a 2nd episode of fUTI had positive result on the DMSA scan performed 3 months after the acute infection. In this study, the detection rate of cRS at the 2nd episode of fUTI was 2.5%, but it increased to 29.6% at the 3rd episode. Similar to high-grade VUR, cRS seems to be a strong independent predictive factor of recurrent fUTI. Previous renal scarring has already been known as a predictive factor for new renal scar formation [12]. Although a study reported that non-E. coli UTI was associated with the development of renal scars, this study contradicted that result [20].
This study supported the result of a report that CRP could predict renal scarring in children with fUTI [21]. Some studies also reported on the association between serum procalcitonin and development of renal scarring [11,22]. However, the present study could not confirm such an association because blood samples of procalcitonin were not obtained from some patients enrolled in this study.
A study previously reported that the CRP values and uNa/K in infants could be useful tools for discriminating between pyelonephritis and other fUTI (pyelitis, lower UTI with other fever focus) [16]. According to the study, high CRP values and low uNa/K among infants with fUTI had suggested the increased possibility of the presence of cortical defects on their acute DMSA scan. Another study summarized the hypothetical pathogenesis of acute pyelonephritis related to the changes of CRP or uNa/K [18]. Renal parenchymal inflammation due to ascending bacterial infection can cause changes in the diameter of glomerular capillaries, which can activate the intrarenal renin-angiotensin-aldosterone pathway, thus resulting in the antinatriuretic phenomenon [18]. Similarly, the present study shows that the DMSA-positive group had significantly higher CRP and lower uNa/K than the control group (Table 1). Hence, this study suggests that high CRPs and low uNa/K in infants with repetitive fUTI may suggest an increased possibility of the presence of RPDs, whereas low CRPs and high uNa/K may suggest a decreased possibility of the presence of RPDs. The cutoff values of those factors in this study were not calculated because of the small sample size. In the previous report [16], the values deduced to discriminate acute pyelonephritis from other fUTI were CRP level of 3.0 mg/dL and uNa/K of 1.015.
Obstructive uropathy and congenital hypoplastic kidney are the common diseases that cause CRI in children [23]. Therefore, children with congenital hypoplastic kidney should be monitored throughout their lifetime. It is usually discovered in children in their early life through an US or DMSA scan of fUTI or recurrent fUTI. Thus, a DMSA scan should be done regardless of previous VCUG, particularly in children with recurrent fUTI. We believe that early detection and treatment of children with recurrent fUTI who have the potential risk of CRI would be helpful in preventing the progression into CRI.
This study has some limitations. The number of enrolled patients is small, and the follow-up period is very short. The retrospective nature of the study has many statistical limitations and biases that preclude a concrete conclusion. Although most of the DMSA scans after the 3rd episode of fUTI showed RPD, there were difficulties in differentiating whether this was due to acute inflammation or due to renal scarring. A follow-up scan could not be performed because most patients continued treatment at a tertiary hospital.
In conclusion, RPDs on the DMSA scan were found more frequently in infants with recurrent fUTI than in the control group. High CRP values and low uNa/K sampled at the acute phase of infection were helpful in predicting the presence of RPDs in infants with recurrent fUTI.
Notes
References
1. Chang SL, Shortliffe LD. Pediatric urinary tract infections. Pediatr Clin North Am. 2006; 53:379–400.

2. Scott Wang HH, Cahill D, Panagides J, Logvinenko T, Nelson C. Top-down versus bottom-up approach in children presenting with urinary tract infection: comparative effectiveness analysis using RIVUR and CUTIE data. J Urol. 2021; 206:1284–90.

3. O'Reilly SE, Plyku D, Sgouros G, Fahey FH, Ted Treves S, Frey EC, et al. A risk index for pediatric patients undergoing diagnostic imaging with (99m)Tc-dimercaptosuccinic acid that accounts for body habitus. Phys Med Biol. 2016; 61:2319–32.
4. Subcommittee on Urinary Tract Infection; Steering Committee on Quality Improvement and Management, Roberts KB. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011; 128:595–610.

5. Cain JE, Di Giovanni V, Smeeton J, Rosenblum ND. Genetics of renal hypoplasia: insights into the mechanisms controlling nephron endowment. Pediatr Res. 2010; 68:91–8.

6. Bonsib SM. Renal hypoplasia, from grossly insufficient to not quite enough: consideration for expanded concepts based upon the author's perspective with historical review. Adv Anat Pathol. 2020; 27:311–30.

7. Abdelhalim A, Khoury AE. Critical appraisal of the top-down approach for vesicoureteral reflux. Investig Clin Urol. 2017; 58(Suppl 1):S14–22.

8. Al Qahtani W, Sarhan O, Al Otay A, El Helaly A, Al Kawai F. Primary bilateral high-grade vesicoureteral reflux in children: management perspective. Cureus. 2020; 12:e12266.

9. Lee JH, Rhie S. Reconsideration of urine culture for the diagnosis of acute pyelonephritis in children: a new challenging method for diagnosing acute pyelonephritis. Korean J Pediatr. 2019; 62:433–7.

10. Park YS. Renal scar formation after urinary tract infection in children. Korean J Pediatr. 2012; 55:367–70.

11. Leroy S, Fernandez-Lopez A, Nikfar R, Romanello C, Bouissou F, Gervaix A, et al. Association of procalcitonin with acute pyelonephritis and renal scars in pediatric UTI. Pediatrics. 2013; 131:870–9.

12. Soylu A, Demir BK, Turkmen M, Bekem O, Saygi M, Cakmakci H, et al. Predictors of renal scar in children with urinary infection and vesicoureteral reflux. Pediatr Nephrol. 2008; 23:2227–32.

13. Becerir T, Yuksel S, Evrengul H, Ergin A, Enli Y. Urinary excretion of pentraxin-3 correlates with the presence of renal scar following acute pyelonephritis in children. Int Urol Nephrol. 2019; 51:571–7.

14. Lee JH, Yim HE, Yoo KH. Associations of plasma neutrophil gelatinase-associated lipocalin, anemia, and renal scarring in children with febrile urinary tract infections. J Korean Med Sci. 2020; 35:e65.

15. Gokce I, Alpay H, Biyikli N, Unluguzel G, Dede F, Topuzoglu A. Urinary levels of interleukin-6 and interleukin-8 in patients with vesicoureteral reflux and renal parenchymal scar. Pediatr Nephrol. 2010; 25:905–12.

16. Jung SJ, Lee JH. Prediction of cortical defect using C-reactive protein and urine sodium to potassium ratio in infants with febrile urinary tract infection. Yonsei Med J. 2016; 57:103–10.

17. Lee JH. Discrimination of culture negative pyelonephritis in children with suspected febrile urinary tract infection and negative urine culture results. J Microbiol Immunol Infect. 2019; 52:598–603.

18. Lee JH, Jang SJ, Rhie S. Antinatriuretic phenomena seen in children with acute pyelonephritis may be related to the activation of intrarenal RAAS. Medicine (Baltimore). 2018; 97:e12152.

19. Kosmeri C, Kalaitzidis R, Siomou E. An update on renal scarring after urinary tract infection in children: what are the risk factors? J Pediatr Urol. 2019; 15:598–603.

20. Shaikh N, Craig JC, Rovers MM, Da Dalt L, Gardikis S, Hoberman A, et al. Identification of children and adolescents at risk for renal scarring after a first urinary tract infection: a meta-analysis with individual patient data. JAMA Pediatr. 2014; 168:893–900.

21. Rodriguez LM, Robles B, Marugan JM, Suarez A, Garcia Ruiz de Morales JM. Do serum C-reactive protein and interleukin-6 predict kidney scarring after urinary tract infection? Indian J Pediatr. 2013; 80:1002–6.

Fig. 1.
Schematic view of our hospital’s diagnostic approach to febrile urinary tract infection (fUTI). KB, kidney and bladder; DMSA, 99mTc-dimercaptosuccinic acid; VCUG, voiding cystourethrography.
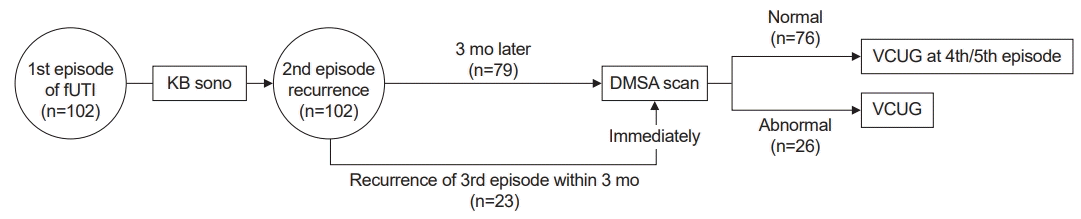
Table 1.
Comparison of data between the DMSA-positive group and controls according to urinary tract imaging strategy
| Variable | DMSA-positive (n=26) | Control (n=76) | P-valuea) |
|---|---|---|---|
| Age (mo) | 4.2±2.1 | 3.5±1.8 | 0.16 |
| Sex, male:female | 24:2 | 55:21 | 0.03 |
| Follow-up (mo) | 10.5±7.6 | 12.2±10.2 | |
| Recurrent APN after DMSA scan | 9 (35) | 10 (13) | 0.01 |
| Interval between the 1st and 2nd episode (mo) | 2.3±2.7 | 3.3±3.1 | 0.13 |
| Urine culture-negative fUTI | 2 (8) | 3 (4) | 0.45 |
| Same bacteria as that in the 1st episode on urine culture | 14 (54) | 52 (68) | 0.16 |
| Non-Escherichia coli cause | 5 (20) | 8 (10) | 0.29 |
| ESBL-positive | 9 (35) | 20 (26) | 0.38 |
| Congenital scar | 9 (35) | 0 | |
| Blood tests | |||
| C-reactive protein (mg/dL) | 7.3±7.7 | 3.7±3.3 | <0.001 |
| White blood cell (/µL) | 18,309±7,744 | 16,043±5,526 | 0.12 |
| Procalcitonin (ng/mL) | 6.1±9.0 | 1.9±2.1 | 0.09 |
| Urine tests | |||
| Protein/creatinine ratio | 1.4±1.9 | 1.8±1.9 | 0.33 |
| Urine Na (mEq/L) | 22.3±16.0 | 28.1±24.6 | 0.07 |
| Urine K (mEq/L) | 34.6±14.0 | 19.0±16.0 | 0.07 |
| Urine Na/K ratio | 0.6±0.4 | 1.1±1.5 | <0.001 |
| Both kidney size discrepancy on US (cm) | 0.7±0.8 | 0.4±0.3 | 0.07 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download