Abstract
Abusive head trauma (AHT) is the most severe form of physical abuse in children. Such injury involves traumatic damage to the head and/or spine of infants and young children. The term AHT was introduced to include a wider range of injury mechanisms, such as intentional direct blow, throw, and even penetrating trauma by perpetuator(s). Currently, it is recommended to replace the former term, shaken baby syndrome, which implicates shaking as the only mechanism, with AHT to include diverse clinical and radiological manifestations. The consequences of AHT cause devastating medical, social and financial burdens on families, communities, and victims. The potential harm of AHT to the developing brain and spinal cord of the victims is tremendous. Many studies have reported that the adverse effects of AHT are various and serious, such as blindness, mental retardation, physical limitation of daily activities and even psychological problems. Therefore, appropriate vigilance for the early recognition and diagnosis of AHT is highly recommended to stop and prevent further injuries. The aim of this review is to summarize the relevant evidence concerning the early recognition and diagnosis of AHT. To recognize this severe type of child abuse early, all health care providers maintain a high index of suspicion and vigilance. Such suspicion can be initiated with careful and thorough history taking and physical examinations. Previously developed clinical prediction rules can be helpful for decision-making regarding starting an investigation when considering meaningful findings. Even the combination of biochemical markers may be useful to predict AHT. For a more confirmative evaluation, neuroradiological imaging is required to find AHT-specific findings. Moreover, timely consultation with ophthalmologists is needed to find a very specific finding, retinal hemorrhage.
Abusive head trauma (AHT) is a term referring to inflicted head and/or spine injury by perpetuator(s). Such intentional injury can cause significant harm to the growing brain and spine of the victims, who are mainly infants and young toddlers. For this reason, the maintenance of a high index of suspicion and early recognition are essential for preventing further injuries. A recent retrospective study investigating the short-term outcomes of surviving AHT clearly showed the adverse effect of AHT. In this study, the following data of 85 children were evaluated : sensation (hearing and vision); gross and fine motor skills; speech and language; cognition; adaptive functioning; behavior; and personal-social skills. The results showed that 56% of the children suffered from moderate or severe disabilities [2] at the 2-year follow-up. The same research group also reported longer term (5 years postinjury) outcomes, and surprisingly, 45 (81.8%) of the 55 followed-up children revealed more than a moderate degree of disabilities [45]. These results suggest that the burden can increase even after a long period. Therefore, the burden of AHT among young children should be regarded as more severe than other unintentional forms of traumatic brain injury (TBI).
Formerly, ‘whiplash shaken infant syndrome’, ‘shaken impact syndrome’, ‘inflicted childhood neurotrauma’ and ‘shaken baby syndrome’ were interchanging terms used to indicate this form of physical abuse [50]. In 1984, Ludwig and Warman [41] introduced the term ‘shaken baby syndrome’ after a review of 20 cases of young children who were believed to be injured only by ‘shaking’. In this study, the victims were believed to be injured not by impact to their heads but by ‘shaking’ only [41]. This theory regarding the injury being fully caused by ‘shaking only’ provoked controversy in medical societies. Whether shaking can only cause significant brain and/or spinal injuries has been studied in some biomechanical studies [13,17], animal models using lambs [21] and even clinical research [1]. Conclusively, based on the reports of these previous studies, the fact that only shaking insult can cause significant brain and/or spinal cord injuries is currently widely accepted [50].
In 2009, the American Academy of Pediatrics (AAP) decided to recommend the term ‘AHT’ over formerly used terms, such as ‘shaken baby syndrome’, because not only shaking but also blunt impact, throw, and/or even penetrating force are causes of AHT. In the policy statement of 2009, the AAP announced that “the use of broad medical terminology that is inclusive of all mechanisms of injury, including shaking” is needed [12]. After this recommendation, the use of the term ‘shaken baby syndrome’ has been discouraged, and inflicted brain and/or spine injury is currently called ‘AHT’.
The aim of this article is to summarize previous relevant studies and recommend a process of early recognition and diagnosis of AHT from a neurosurgical perspective.
In this review, published studies investigating AHT were reviewed to prepare this manuscript. For this purpose, the author carried out a search in PubMed, EMBASE, and Cochrane Library for associated studies through September 2021 using “abusive head trauma”, “shaken baby syndrome”, “physical abuse,” and “child” as search terms. Among the retrieved articles, the relevant studies were selected to prepare this review.
The accurate epidemiology of AHT is uncertain in many countries, including Korea. The problem of a lack of reporting or underreporting of this form of child abuse is associated with the absence of the exact incidence of AHT. Because the main mechanism of AHT, shaking a baby, is usually considered benign activity, some regions worldwide do not consider this activity dangerous to infants. Therefore, in some cultural and social circumstances, AHT is not recorded separately from other forms of abuse.
Despite this limitation, some studies concerning the epidemiology of AHT show that the incidence rates seem to be 20–30 per 100000 children younger than 2 years [19,49]. For example, Keenan et al. [35] collected the data of all North Carolina children younger than 2 years who were admitted to a pediatric intensive care unit (PICU) or died with TBI in 2000 and 2001. The authors estimated the incidence of inflicted brain injury to be 17.0 (95% confidence interval [CI], 13.3–20.7) per 100000 in this age group [19,35]. Furthermore, in a larger study conducted in 15 states in the USA, Eisele et al. [18] found that the incidence rate of nonfatal hospitalization of children (<2 years) was the highest at 1 month of age (178.0 cases per 100000 person-years), followed by a secondary peak at 8 months of age (127.9 cases per 100000 person-years).
Moreover, when considering the results of population-based studies conducted in various countries and regions, the incidence of AHT is approximately 14–53 per 100000 livebirths [4,36,59,60]. In a population-based study conducted in Scotland from 1998 to 1999, the annual incidence of inflicted head injury was 24.6 per 100000 (95% CI, 14.9–38.5) [3].
Careful history-taking is the first step in the early recognition and diagnostic process of AHT. Usually, nonverbal infants or toddlers are the victims of AHT; therefore, a thorough history can be obtained from the parent(s) or care-takers of the babies. Some suspicious aspects of the history can raise concerns regarding child abuse and neglect. These ‘red flag’ histories are basically ‘medical lies’ and ‘incongruent stories’ regarding trauma events(s) of interest. Some of these aspects are summarized in a recently developed screening tool. The name of the screening tool is ‘FIND’, which is an acronym for “finding instrument of nonaccidental deeds”. According to this screening tool, two or more ‘yes’ responses to the eight questions of the tool indicate highly suspicious cases that should be reported to the police or child protection services. Among the eight questions, the items concerning a suspicious history are as follows [52] : 1. Is there an inappropriate delay in seeking medical help? 2. Does the trauma history change or is it inconsistent among caregivers? 3. Are the histories of trauma (event) impossible considering the developmental capabilities of the child? 4. Are the mechanism and type of injury incompatible/conflicting with the wound(s)? 5. Is the injury clinically significant for an infant (<2 years)? Additionally, the provision of no history or a trivial trauma mechanism for a significant injury is an important clue for suspecting inflicted injury, including AHT.
The presenting symptoms of AHT can vary from subtle ‘irritability’ to the most serious ‘status epilepticus’. As only mild symptoms, such as fussiness and drowsiness, can be presenting symptoms of AHT, health care providers should maintain a high index of suspicion. Severe symptoms, such as seizure and apnea, with the provision of a relatively mild injury mechanism, such as slipping on the bath floor, should raise suspicion regarding child abuse, including AHT [56,58,65].
A physical examination from the scalp to the tip of the toe should be completed for suspected cases. The most common visible sign on the skin is bruising. Many studies have attempted to find characteristic features of bruising caused by physical abuse. In the late 1980s, Hanigan et al. [26] introduced ‘tin ear syndrome’, which sometimes implies a symmetrical, tin-colored discoloration wound (bruise) in the ear, highly suggesting pinch insult to the ear. The authors found that this type of skin bruising is closely related to pediatric head injuries, such as subdural hemorrhage (SDH) [26]. After this study, many researchers conducted studies concerning suspicious bruising specific to child abuse [29,39,42], including the baby manikin experiment model [16].
Collectively, the ‘TEN-4 FACESp rule’ is currently accepted as a helpful tool in prehospital and hospital settings. The alphabet of the rule is an acronym for the suspicious body parts of bruising, i.e., torso, ear(s), and neck for TEN, in the original study [54]. The additional FACESp implies high-risk body parts from further studies, such as the frenulum, angle of the jaw, cheek, sclera and patterned wound. The number 4 of the rule indicates the important age by twofold. First, bruising on ‘TEN FACESp’ body parts should be suspected as a sign of physical abuse in children younger than 4 years. Second, any bruise found in infants younger than 4 months should be given careful attention in terms of possible abuse. As a normal developmental milestone, infants can usually flip themselves at the age of only 4 months as the first step of mobilization; therefore, babies younger than 4 months cannot injure themselves by any movement. This rule was introduced in the early 2010s [54] and recently validated with sensitivity 95.6% (95% CI, 93.0–97.3%) and specificity 87.1% (95% CI, 85.4–88.6%) in a larger scale [55].
A recent study showed that a significant proportion of young children with suspicious bruising was finally confirmed as victims of physical abuse. According to Crumm et al.’s research [15] published in 2021, among the 43771 visitors to emergency departments who were screened for bruising, 163 (0.4%) had high-risk bruising according to the TEN4 FACESp rule. The rates of likely or definite abuse were 50% and 28% in children aged under 6 months and 6 months to 4 years, respectively. These findings emphasize the importance of minor wounds, such as bruising in abnormal regions of the body, for starting an investigation of possible child abuse. Moreover, in this study, the results showed that of 48 children aged 6 months or younger, 30 (63%) children underwent head computed tomography (CT) scans, and seven of these 30 (23%) children exhibited intracranial or head injury [15].
RH is also known to be a significant presentation of AHT. An early study by Buys et al. [8] in the 1990s showed the following impressive results : no RH was found in the accidental head injury group, and 100% positive RH was found in the nonaccidental head injury group. Along with these results, a systematic review was published in 2013. The review showed that RHs are found in 78% of AHT victims and that the odds ratio of AHT is 14.7 (95% CI, 6.39–33.62) compared to that in children without RH. In this systematic review, the authors summarized the characteristic features of RH specific for AHT. These findings are bilateral RH, too many RHs to count, and RH extending to the periphery (to the ora serrata) (Fig. 1) [44]. In another review, more detailed but rare findings, such as retinoschisis (break in the retina), are also considered very specific to AHT [28].
As RH is a very specific finding of AHT, timely evaluation is recommended. According to a recent review, the appropriate timing for an examination of the retina to avoid spontaneous resolution is recommended preferably within 24 hours after presentation [28]. Although RH is very specific to AHT, the examination is necessarily required only in the case of abnormal brain imaging. In 2010, Thackeray et al. [62] showed that among 282 children with no evidence of TBI on neuroimaging, only two (0.7%; 95% CI, 0.1–2.5) children had RH. In another study by Burkhart et al. [7], the results were similar as follows: among the 168 children enrolled, 23 children (13.7%) were found to have skull or nonskull fracture without intracranial hemorrhage, and among these children, no RH was found. These findings indicate that an RH evaluation is only indicated in the presence of intracranial injury [7].
CPRs are usually constructed based on a combination of clinical variables that are statistically associated with certain conditions. The aim of CPRs is mainly to help decision-making in the diagnosis and even prognostication of patients with certain pathologic conditions. For AHT, some CPRs have been developed and introduced [53].
The Predicting Abusive Head Trauma (PredAHT) CPR uses six influential features (RH, rib and long-bone fractures, apnea, seizures and head or neck bruising) to predict the presence of AHT [43]. In a validation study of PredAHT, the authors found that when more than three variables were positive, the estimated probability of AHT was higher than 81.5%, with a sensitivity of 72.3% (95% CI, 60.4–81.7), specificity of 85.7% (95% CI, 78.8–90.7), and area under the curve of 0.88 (95% CI, 0.823–0.926) [14].
To help decision-making regarding using CT of the brain in suspicious patients at emergency departments, the Pittsburgh infant brain injury score (PIBIS) was developed and validated. The CPR subjects were well-appearing infants (younger than 1 year) without fever (body temperature <38.3°C), no history of trauma, and central nervous symptoms, such as drowsiness or irritability. The criteria for the score are as follows : presence of abnormal skin exam (bruising), age >3 months, head circumference >85th percentile, and serum hemoglobin <11.2 g/dL. The authors assigned weighted point(s) to the four criteria by a logistic regression model (Table 1). The validation studies of the score showed that if the score was 2 or greater, the sensitivity and specificity of the score were 93.3% (95% CI, 89.0–96.3) and 53% (95% CI, 49.3–57.1), respectively [5]. Therefore, when an infant younger than one year is suspected to have AHT, we can calculate the PIBS using a careful physical examination and simple laboratory study (complete blood count) to make decisions regarding brain CT utilization.
In 2013, the Pediatric Brain Injury Network (PediBIRN) derived a CPR for patients admitted to the PICU. The aim of this CPR is to identify children who could benefit from an evaluation of probable child abuse. The four criteria of this CPR are as follows : acute respiratory compromise before admission, bruising of the ear, torso, or neck (TEN-4), bilateral or interhemispheric SDH, and any skull fractures other than a linear, unilateral, isolated, nondiastatic, or parietal fracture. Using only these four criteria, a validation study found that the PediBIRN score identified 98% of PICU patients ultimately diagnosed with AHT [33].
Recently, PediBIRN was externally validated in both the PICU and a general ward-admitting setting in Australia and New Zealand. In this validation study, the PediBIRN CPR showed 96% sensitivity among all admitted patients and 100% sensitivity among patients admitted to the PICU [30]. However, as a trade-off of the high sensitivity, the specificity is quite low. The specificity results for PICU admissions and all admissions were 11% (95% CI, 0–48) and 43% (95% CI, 32–53), respectively. The low specificity is believed to mainly originate from the skull fracture criteria.
Moreover, an updated version of the original PediBIRN with more than seven criteria, PediBIRN-7 (Table 2), was also introduced and awaits external validation. The new PediBIRN-7 incorporates the results of AHT evaluations (skeletal survey, neuroimaging, and a retinal exam) and can be applied to acutely head-injured children younger than 3 years who are hospitalized for intensive care [32]. If any of the PediBIRN-7 criteria are met in infants or young children admitted to an intensive care setting, health care providers should seriously consider a child abuse investigation.
In 2017, a combination of biochemical markers was introduced as a CPR to predict intracranial hemorrhage. The CPR was named the Biomarker of infant brain injury score (BIBIS) and used a binary multivariable logistic regression model with three serum biochemical markers (matrix metallopeptidase-9, neuron-specific enolase, and vascular cellular adhesion molecule-1) and one clinical variable (total hemoglobin). The CPR showed a sensitivity of 89.3% (95% CI, 87.7–90.4) and a specificity of 48.0% (95% CI, 47.3–48.9) in predicting acute intracranial hemorrhage. Although this study considered experimental biomarkers and is not designed for AHT, the rule suggests that the combination of biochemical markers can be useful in predicting intracranial hemorrhage in suspicious AHT cases. The positive and negative predictive values of the CPR were 21.3% and 95.6%, respectively [6].
The key component of the diagnosis of AHT is radiologic evaluations of the brain and/or spine. Radiologically important findings can be divided into vascular injury (intracranial hemorrhage), parenchymal lesions and spine injuries.
The most important vascular injury form in AHT victims is SDH. In an early study, Hymel et al. [31] compared the cranial CT scans of 39 children suffering from AHT with those of 39 matched children as a control group. This comparative study found that the more common findings in the AHT group were SDH, interhemispheric falx hemorrhage, large (nonacute) extra-axial fluid, and basal ganglia edema [31]. Reece and Sege [57] also published another classical comparative study in 2000 comparing an accident group (81% of the subjects) and a definite abuse group (19%). The major difference was the prevalence of SDH, which was 10% in the accident group and 46% in the definite abuse group [57]. According to a more comprehensive systematic review of 21 previous studies, the neuroradiologic features distinguishing AHT from other TBIs are multiple SDH over convexity, interhemispheric hemorrhages, posterior fossa SDH, hypoxic ischemic injury (HII) and cerebral edema [38]. Moreover, in a 20-year comparative study of children aged under 2 years conducted in Auckland, New Zealand, the authors found that the odds ratio of SDH in the AHT group was 23.6 (95% CI, 11.1–50.4) compared with the accidental TBI group [37]. Conclusively, SDH is the most common neuroradiologic finding in AHT and is sometimes bilateral or multiple [9].
Mixed density SDH on CT scan is also an important characteristic of AHT patients, which may indicate the repetitive, different timing of the causative insults. In a prospective study enrolling 66 children with SDH from 1995 to 1998, the results showed that chronic or mixed acute and chronic SDH were found only in abused children and not in any nonintentional injury group [20]. Another comparative study also showed that homogeneous SDH was more frequent in accidental TBI than AHT (74% vs. 33%) and that mixed-density SDH was significantly more common in cases of nonaccidental head injury (67% vs. 18%) [64]. These findings indicate that mixed attenuation SDH does not exclusively occur in AHT and that mixed SDH can be the result of accidental trauma. Generally, mixed-attenuation SDHs can occur in the following four possible situations : coexistence of acute and hyperacute hemorrhage, acute hematoma with sedimentation, mixed form of acute blood with cerebrospinal fluid, and episodic, acute or chronic hemorrhage [25]. Therefore, mixed, multistage SDH is not a pathognomonic sign of AHT; however, this finding is very helpful for further investigation of suspected child abuse cases (Fig. 2).
One of the most common controversies regarding SDH in AHT cases is associated with benign enlargement of the subarachnoid space (BESS). Because an early study indicated that even minor trauma can cause SDH in BESS patients [48], some argue that SDH in BESS patients should not be regarded as evidence of AHT. This opinion can be easily heard in medical conferences as personal experience and even in the court of law as a significant debate. However, a study clearly proved that SDH is rarely found even in BESS patients (4/177, 2.3%) [47]. Additionally, according to the consensus statement of many radiologic societies worldwide (Europe and the USA), the true incidence of SDH in BESS in the published literature is less than 6%, and most published studies concerning SDH in BESS cases have flaws due to the incomplete assessment of child abuse [11]. Therefore, even in BESS patients, an appropriate evaluation of AHT should be started if the patient has SDH. In another study concerning SDH in BESS patients, 34 of 149 (22.8%) patients had SDH and BESS, and among the 34 BESS patients, 17 patients (50%) had other suspicious findings of AHT. The authors concluded that even BESS patients with SDH benefit from a full evaluation of child abuse [27].
Diffuse parenchymal injury per se is not a specific finding of AHT; however, a diffuse form of parenchymal injury with accompanying SDH can be regarded as a characteristic of AHT [38,56,66]. Because diffuse parenchymal injury is caused by only a high-force mechanism, such as a motor vehicle crash or fall from higher ground, diffuse parenchymal injury with provision of minor head trauma, such as a short fall or slip-down, should raise concerns regarding AHT [63]. Some studies have indicated that the predominant parenchymal injury in AHT is HII [23,46]. In a systematic review by Kemp et al. [38], the diffuse parenchymal lesions specific to AHT were HII (odds ratio [OR], 3.7; 95% CI, 1.4–10.0) and cerebral edema (OR, 2.2; 95% CI, 1.0–4.5) [51].
Early parenchymal injury, such as HII, is better visualized by magnetic resonance imaging (MRI). Diffusion-weighted MRI imaging can visualize dramatic cytotoxic injury, which is commonly observed as a watershed pattern of diffusion restriction in most AHT cases [61,67]. The importance of parenchymal injury is the association with the outcome of AHT. In a study investigating various neuroimaging findings of AHT children, Gencturk et al. [24] showed that the presence of HII was significantly correlated with the clinical outcome (p=0.017).
Until the recent reports of spinal injuries in AHT victims, spine imaging was not routinely considered in the evaluation of AHT patients. However, accumulating evidence of posterior ligamentous injury in the cervical spine suggests the requirement for a spine evaluation in suspicious AHT cases [10,34]. Moreover, spinal SDH was recently recognized as an important finding of AHT [22]. As spinal SDH is extremely rare in conventional TBI cases, the presence of spinal SDH highly suggests the possibility of AHT. In a small retrospective study, spinal SDH accompanying SDH was common in AHT patients (8/18 cases, 44%), which were all clinically occult [40]. Most spinal SDHs are found in the thoracolumbar area and are associated with posterior fossa SDH, which may not be connected to the spinal subdural blood [25].
The origin of spinal SDH is still controversial. A recent review suggests that high-power shaking in AHT cases damages the myodural band that connects the suboccipital structures to the dura and consequently causes secondary cleavage of the dura and a shift of the intracranial SDH to the spinal subdural space [51].
AHT causes significant medical, social, and financial burdens on families, communities and victims. To recognize this severe type of child abuse early, all health care providers maintain a high index of suspicion and vigilance for appropriate reporting and evaluation. The suspicion can be initiated with careful and thorough history taking and physical examinations, such as other forms of TBI and medical conditions.
Previously developed CPRs can be helpful in decision-making regarding starting an investigation when considering meaningful findings (PredHAT), emergency departments (PIBIS), and wards and intensive care units (PediBIRN). Even a combination of biochemical markers may be useful in predicting AHT (BIBIS).
For a more confirmative evaluation, neuroradiological imaging is required to find AHT-specific findings, such as SDH, HII and spinal injuries. Moreover, timely consultation with ophthalmologists is needed to find a very specific finding, RH.
Notes
References
1. Adamsbaum C, Grabar S, Mejean N, Rey-Salmon C. Abusive head trauma: judicial admissions highlight violent and repetitive shaking. Pediatrics. 126:546–555. 2010.

2. Badger S, Waugh MC, Hancock J, Marks S, Oakley K. Short term outcomes of children with abusive head trauma two years post injury: a retrospective study. J Pediatr Rehabil Med. 13:241–253. 2020.

3. Barlow KM, Minns RA. Annual incidence of shaken impact syndrome in young children. Lancet. 356:1571–1572. 2000.

4. Bennett S, Ward M, Moreau K, Fortin G, King J, Mackay M, et al. Head injury secondary to suspected child maltreatment: results of a prospective canadian national surveillance program. Child Abuse Negl. 35:930–936. 2011.

5. Berger RP, Fromkin J, Herman B, Pierce MC, Saladino RA, Flom L, et al. Validation of the Pittsburgh infant brain injury score for abusive head trauma. Pediatrics. 138:e20153756. 2016.

6. Berger RP, Pak BJ, Kolesnikova MD, Fromkin J, Saladino R, Herman BE, et al. Derivation and validation of a serum biomarker panel to identify infants with acute intracranial hemorrhage. JAMA Pediatr. 171:e170429. 2017.

7. Burkhart ZN, Thurber CJ, Chuang AZ, Kumar KS, Davis GH, Kellaway J. Risk factors associated with retinal hemorrhage in suspected abusive head trauma. J AAPOS. 19:119–123. 2015.

8. Buys YM, Levin AV, Enzenauer RW, Elder JE, Letourneau MA, Humphreys RP, et al. Retinal findings after head trauma in infants and young children. Ophthalmology. 99:1718–1723. 1992.

9. Case ME. Distinguishing accidental from inflicted head trauma at autopsy. Pediatr Radiol. 44:S632–S640. 2014.

10. Choudhary AK, Ishak R, Zacharia TT, Dias MS. Imaging of spinal injury in abusive head trauma: a retrospective study. Pediatr Radiol. 44:1130–1140. 2014.

11. Choudhary AK, Servaes S, Slovis TL, Palusci VJ, Hedlund GL, Narang SK, et al. Consensus statement on abusive head trauma in infants and young children. Pediatr Radiol. 48:1048–1065. 2018.

12. Christian CW, Block R. Abusive head trauma in infants and children. Pediatrics. 123:1409–1411. 2009.

13. Cory CZ, Jones BM. Can shaking alone cause fatal brain injury? A biomechanical assessment of the Duhaime shaken baby syndrome model. Med Sci Law. 43:317–333. 2003.
14. Cowley LE, Morris CB, Maguire SA, Farewell DM, Kemp AM. Validation of a prediction tool for abusive head trauma. Pediatrics. 136:290–298. 2015.

15. Crumm CE, Brown ECB, Thomas-Smith S, Yu DTY, Metz JB, Feldman KW. Evaluation of an emergency department high-risk bruising screening protocol. Pediatrics. 147:e2020002444. 2021.

16. Dsouza R, Bertocci G. Impact sites representing potential bruising locations associated with bed falls in children. Forensic Sci Int. 286:86–95. 2018.

17. Duhaime AC, Christian CW, Rorke LB, Zimmerman RA. Nonaccidental head injury in infants--the “shaken-baby syndrome”. N Engl J Med. 338:1822–1829. 1998.

18. Eisele JA, Kegler SR, Trent RB, Coronado VG. Nonfatal traumatic brain injury-related hospitalization in very young children-15 states, 1999. J Head Trauma Rehabil. 21:537–543. 2006.

19. Ellingson KD, Leventhal JM, Weiss HB. Using hospital discharge data to track inflicted traumatic brain injury. Am J Prev Med. 34:S157–S162. 2008.

20. Feldman KW, Bethel R, Shugerman RP, Grossman DC, Grady MS, Ellenbogen RG. The cause of infant and toddler subdural hemorrhage: a prospective study. Pediatrics. 108:636–646. 2001.

21. Finnie JW, Blumbergs PC, Manavis J, Turner RJ, Helps S, Vink R, et al. Neuropathological changes in a lamb model of non-accidental head injury (the shaken baby syndrome). J Clin Neurosci. 19:1159–1164. 2012.

22. Garcia-Pires F, Jayappa S, Desai S, Ramakrishnaiah RH, Choudhary AK. Spinal subdural hemorrhage in abusive head trauma: a pictorial review. Pediatr Radiol. 51:980–990. 2021.

23. Geddes JF, Hackshaw AK, Vowles GH, Nickols CD, Whitwell HL. Neuropathology of inflicted head injury in children. I. Patterns of brain damage. Brain. 124:1290–1298. 2001.

24. Gencturk M, Tore HG, Nascene DR, Zhang L, Koksel Y, McKinney AM. Various cranial and orbital imaging findings in pediatric abusive and non-abusive head trauma, and relation to outcomes. Clin Neuroradiol. 29:253–261. 2019.

25. Gunda D, Cornwell BO, Dahmoush HM, Jazbeh S, Alleman AM. Pediatric central nervous system imaging of nonaccidental trauma: beyond subdural hematomas. Radiographics. 39:213–228. 2019.

26. Hanigan WC, Peterson RA, Njus G. Tin ear syndrome: rotational acceleration in pediatric head injuries. Pediatrics. 80:618–622. 1987.
27. Hansen JB, Frazier T, Moffatt M, Zinkus T, Anderst JD. Evaluations for abuse in young children with subdural hemorrhages: findings based on symptom severity and benign enlargement of the subarachnoid spaces. J Neurosurg Pediatr. 21:31–37. 2018.

28. Hansen JB, Killough EF, Moffatt ME, Knapp JF. Retinal hemorrhages: abusive head trauma or not? Pediatr Emerg Care. 34:665–670. 2018.
29. Hibberd O, Nuttall D, Watson RE, Watkins WJ, Kemp AM, Maguire S. Childhood bruising distribution observed from eight mechanisms of unintentional injury. Arch Dis Child. 102:1103–1109. 2017.

30. Hymel KP, Armijo-Garcia V, Foster R, Frazier TN, Stoiko M, Christie LM, et al. Validation of a clinical prediction rule for pediatric abusive head trauma. Pediatrics. 134:e1537–1544. 2014.

31. Hymel KP, Rumack CM, Hay TC, Strain JD, Jenny C. Comparison of intracranial computed tomographic (CT) findings in pediatric abusive and accidental head trauma. Pediatr Radiol. 27:743–747. 1997.

32. Hymel KP, Wang M, Chinchilli VM, Karst WA, Willson DF, Dias MS, et al. Estimating the probability of abusive head trauma after abuse evaluation. Child Abuse Negl. 88:266–274. 2019.

33. Hymel KP, Willson DF, Boos SC, Pullin DA, Homa K, Lorenz DJ, et al. Derivation of a clinical prediction rule for pediatric abusive head trauma. Pediatr Crit Care Med. 14:210–220. 2013.

34. Jacob R, Cox M, Koral K, Greenwell C, Xi Y, Vinson L, et al. MR imaging of the cervical spine in nonaccidental trauma: a tertiary institution experience. AJNR Am J Neuroradiol. 37:1944–1950. 2016.

35. Keenan HT, Runyan DK, Marshall SW, Nocera MA, Merten DF, Sinal SH. A population-based study of inflicted traumatic brain injury in young children. JAMA. 290:621–626. 2003.

36. Kelly P, Farrant B. Shaken baby syndrome in New Zealand, 2000-2002. J Paediatr Child Health. 44:99–107. 2008.

37. Kelly P, John S, Vincent AL, Reed P. Abusive head trauma and accidental head injury: a 20-year comparative study of referrals to a hospital child protection team. Arch Dis Child. 100:1123–1130. 2015.

38. Kemp AM, Jaspan T, Griffiths J, Stoodley N, Mann MK, Tempest V, et al. Neuroimaging: what neuroradiological features distinguish abusive from non-abusive head trauma? A systematic review. Arch Dis Child. 96:1103–1112. 2011.

39. Kemp AM, Maguire SA, Nuttall D, Collins P, Dunstan F. Bruising in children who are assessed for suspected physical abuse. Arch Dis Child. 99:108–113. 2014.

40. Koumellis P, McConachie NS, Jaspan T. Spinal subdural haematomas in children with non-accidental head injury. Arch Dis Child. 94:216–219. 2009.

41. Ludwig S, Warman M. Shaken baby syndrome: a review of 20 cases. Ann Emerg Med. 13:104–107. 1984.

42. Maguire S, Mann M. Systematic reviews of bruising in relation to child abuse-what have we learnt: an overview of review updates. Evid Based Child Health. 8:255–263. 2013.

43. Maguire SA, Kemp AM, Lumb RC, Farewell DM. Estimating the probability of abusive head trauma: a pooled analysis. Pediatrics. 128:e550–e564. 2011.

44. Maguire SA, Watts PO, Shaw AD, Holden S, Taylor RH, Watkins WJ, et al. Retinal haemorrhages and related findings in abusive and non-abusive head trauma: a systematic review. Eye (Lond). 27:28–36. 2013.

45. Manfield J, Oakley K, Macey JA, Waugh MC. Understanding the five-year outcomes of abusive head trauma in children: a retrospective cohort study. Dev Neurorehabil. 24:361–367. 2021.

46. Matschke J, Büttner A, Bergmann M, Hagel C, Püschel K, Glatzel M. Encephalopathy and death in infants with abusive head trauma is due to hypoxic-ischemic injury following local brain trauma to vital brainstem centers. Int J Legal Med. 129:105–114. 2015.

47. McKeag H, Christian CW, Rubin D, Daymont C, Pollock AN, Wood J. Subdural hemorrhage in pediatric patients with enlargement of the subarachnoid spaces. J Neurosurg Pediatr. 11:438–444. 2013.

48. McNeely PD, Atkinson JD, Saigal G, O’Gorman AM, Farmer JP. Subdural hematomas in infants with benign enlargement of the subarachnoid spaces are not pathognomonic for child abuse. AJNR Am J Neuroradiol. 27:1725–1728. 2006.
49. Minns RA, Jones PA, Mok JY. Annual incidence of shaken impact syndrome in young children. Am J Prev Med. 34:S126–S133. 2008.
50. Narang SK, Fingarson A, Lukefahr J, Council on Child Abuse. Abusive head trauma in infants and children. Pediatrics. 145:e20200203. 2020.

51. Oates AJ, Sidpra J, Mankad K. Parenchymal brain injuries in abusive head trauma. Pediatr Radiol. 51:898–910. 2021.

52. Paek SH, Jung JH, Kwak YH, Kim DK, Ryu JM, Noh H, et al. Development of screening tool for child abuse in the Korean emergency department: using modified Delphi study. Medicine (Baltimore). 97:e13724. 2018.
53. Pfeiffer H, Crowe L, Kemp AM, Cowley LE, Smith AS, Babl FE, et al. Clinical prediction rules for abusive head trauma: a systematic review. Arch Dis Child. 103:776–783. 2018.

54. Pierce MC, Kaczor K, Aldridge S, O’Flynn J, Lorenz DJ. Bruising characteristics discriminating physical child abuse from accidental trauma. Pediatrics. 125:67–74. 2010.

55. Pierce MC, Kaczor K, Lorenz DJ, Bertocci G, Fingarson AK, Makoroff K, et al. Validation of a clinical decision rule to predict abuse in young children based on bruising characteristics. JAMA Netw Open. 4:e215832. 2021.

56. Piteau SJ, Ward MG, Barrowman NJ, Plint AC. Clinical and radiographic characteristics associated with abusive and nonabusive head trauma: a systematic review. Pediatrics. 130:315–323. 2012.

57. Reece RM, Sege R. Childhood head injuries: accidental or inflicted? Arch Pediatr Adolesc Med. 154:11–15. 2000.
58. Roach JP, Acker SN, Bensard DD, Sirotnak AP, Karrer FM, Partrick DA. Head injury pattern in children can help differentiate accidental from non-accidental trauma. Pediatr Surg Int. 30:1103–1106. 2014.

59. Shanahan ME, Zolotor AJ, Parrish JW, Barr RG, Runyan DK. National, regional, and state abusive head trauma: application of the CDC algorithm. Pediatrics. 132:e1546–e1553. 2013.

60. Sibert JR, Payne EH, Kemp AM, Barber M, Rolfe K, Morgan RJ, et al. The incidence of severe physical child abuse in wales. Child Abuse Negl. 26:267–276. 2002.

61. Suh DY, Davis PC, Hopkins KL, Fajman NN, Mapstone TB. Nonaccidental pediatric head injury: diffusion-weighted imaging findings. Neurosurgery. 49:309–318. 2001.

62. Thackeray JD, Scribano PV, Lindberg DM. Yield of retinal examination in suspected physical abuse with normal neuroimaging. Pediatrics. 125:e1066–e1071. 2010.

63. Thomas AG, Hegde SV, Dineen RA, Jaspan T. Patterns of accidental craniocerebral injury occurring in early childhood. Arch Dis Child. 98:787–792. 2013.

64. Tung GA, Kumar M, Richardson RC, Jenny C, Brown WD. Comparison of accidental and nonaccidental traumatic head injury in children on noncontrast computed tomography. Pediatrics. 118:626–633. 2006.
Fig. 1.
Retinal examination of a 7 months old girl (left eye). Vitreous hemorrhage with multiple, multilayer (different-shaped) retinal hemorrhages are observed. The parents provided no relevant trauma history.
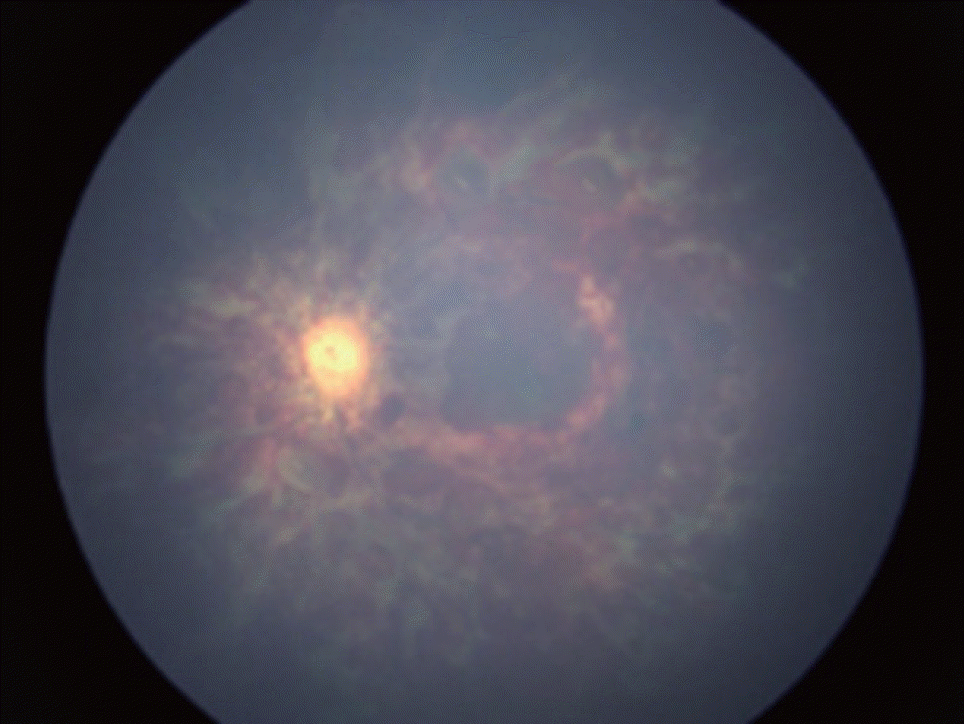
Fig. 2.
Brain computed tomography of an 8 months old boy. Multistage subdural hemorrhage (SDH) is observed such as 2.8 cm (width) acute stage SDH in left frontal convexity (A), with diffuse iso-attenuation lesion in left convexity, suspicious subacute stage SDHs (B). The parents provided only trivial trauma events.
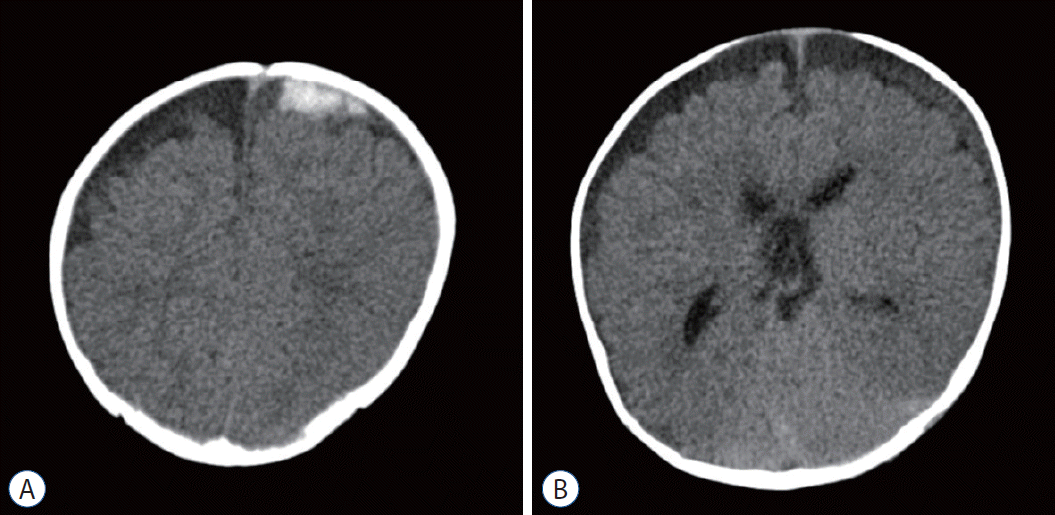
Table 1.
Pittsburgh Infant Brain Injury Score (PIBIS) criteria [5]
| Variable | Point |
|---|---|
| Abnormality on dermatologic examination | 2 |
| Age ≥3.0 month | 1 |
| Head circumference >85th percentile | 1 |
| Hemoglobin <11.2 g/dL | 1 |
Table 2.
The PediBIRN-7 : seven variables used to estimate AHT probability [32]




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download