Abstract
Traumatic brain injury (TBI) is a major public health issue that causes significant morbidity and mortality in the pediatric population. Pediatric minor TBIs are the most common and are widely underreported because not all patients seek medical attention. The specific management of these patients is distinct from that of adult patients because of the different physiologies in these age groups. This article focuses on minor TBIs, particularly growing skull fractures, traumatic cerebrospinal fluid leakage, and concussion.
Pediatric traumatic brain injury (TBI) remains a major public health concern and causes significant morbidity and mortality. With the advancement of social life, minor pediatric TBI occurs more frequently than ever [6]. Since pediatric minor TBI differs from adult minor TBI in terms of the mechanism of trauma, prognosis, and mortality, it is important to identify the characteristics of pediatric minor TBI for its proper treatment and prognosis.
In this article, literature on growing skull fracture (GSF), traumatic cerebrospinal fluid (CSF) leakage, and concussion, which are representative diseases among pediatric minor brain injuries, is reviewed.
GSF is a rare complication that occurs in infants and children, first described clinically by Howship in 1816. It has an incidence of approximately 0.05% to 1.6%. In addition, more than 90% of these GSFs occur under 3 years of age, and most occur chronically [19].
The pathogenesis of GSF is skull fracture with dura mater tearing accompanied by arachnoid adhesions. The pulsatile force of the brain during growth causes the fracture in the thin skull to enlarge. This interposition of herniated elements prevents osteoblasts from migrating to the fracture site, inhibiting healing. The resorption of the adjacent bone by the continuous pressure from tissue herniation through the bone gap adds to the progression of the fracture line [1,13,19,29]. The parietal bone is the most common site of GSF, followed by the occipital and frontal bones.
Several studies have reported a diagnosis of GSF [18,24,33], and early diagnosis is the most important. In the early stage of GSF, clinical presentations and radiological examinations are the most useful diagnostic clues. Common clinical presentations of GSF are pulsatile scalp swelling that grows when a child cries, headache, epileptic seizures, and neurological deficits [7].
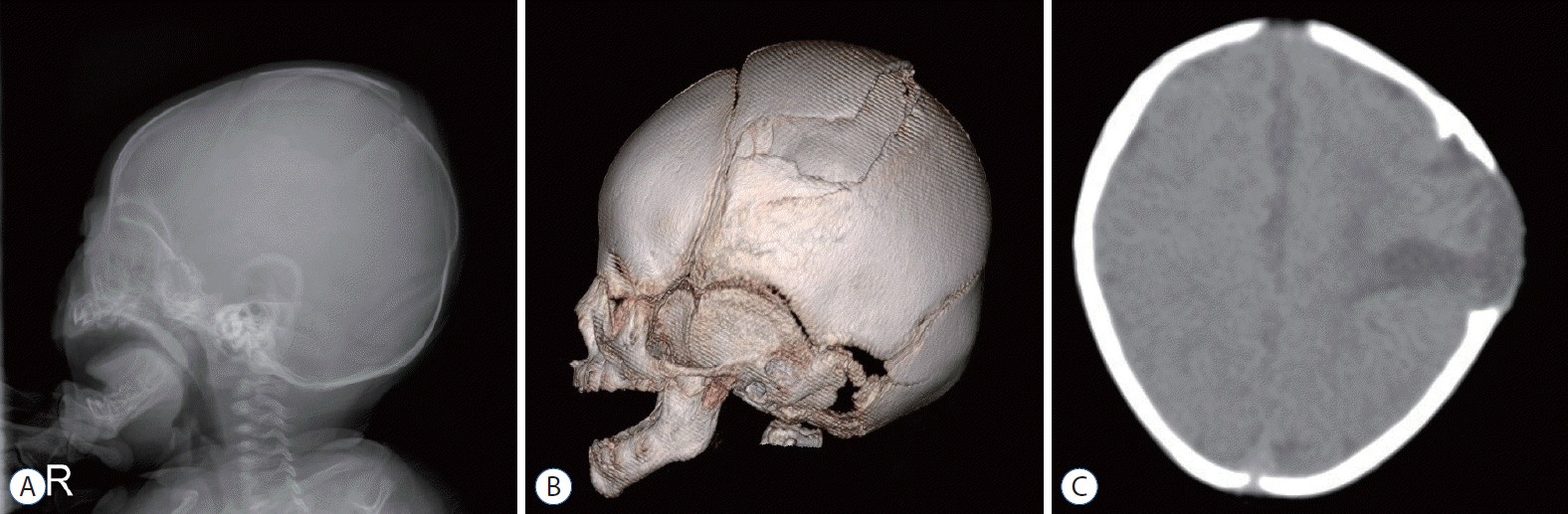
Radiological diagnosis of GSF is mostly possible with simple radiography and computed tomography (CT) (Fig. 1); however, if necessary, magnetic resonance imaging (MRI) may be needed.
Simple radiographs of GSF usually show an enlarged diastatic fracture and linear lytic lesions in the skull with scalloped margins. CT demonstrates the same bony abnormalities and evidence of injury in the underlying brain during the early period, as well as leptomeningeal cyst, ventricular asymmetry, focal dilatation of the lateral ventricle, and herniation of the brain tissue into the bony defect.
However, these diagnostic methods are not useful for diagnosing early-stage GSF. In such cases, MRI shows herniated brain tissue through the skull defect, as well as a leptomeningeal cyst or encephalomalacia [10,14,33].
The treatment of GSF involves surgical repair of the dura mater tear and cranioplasty. In the treatment of GSF, surgery should be performed to prevent further brain damage, and the most important technique is watertight closure of the dura mater [23,31], which includes removal of the leptomeningeal cyst and encephalomalacia. If the dural defect is wide, fascia lata, cadaveric dural grafts, and artificial dura are often used. For cranioplasty, autologous bone is mainly used, and an alloplastic material (polymethyl methacrylate, titanium) can be used when the skull defect is wide [31].
The operator should be careful not to remove functional brain tissue while removing the exposed brain through the skull defect during surgery. If surgery is performed with epileptic seizures, electrophysiological monitoring should be performed during the removal of lesions that cause epileptic seizures. Some authors have reported that a ventriculoperitoneal shunt operation is often required after surgery to prevent hydrocephalus or CSF leakage.
In conclusion, early diagnosis and treatment of GSF are important in preventing the progression of neurological deficits and cranial deformities. In particular, even if only a simple skull fracture is seen in an infant, it is recommended to fully explain the possibility of progression to GSF to the parent, and to conduct a physical examination and imaging study follow-up for 3 months after the initial head trauma.
Traumatic CSF leakage occurs in 2% of all pediatric TBIs and in 12–30% of cases with skull base fractures and is most common in calcaneal fractures of the frontal sinus, ethmoid sinus, and temporal bone. Traumatic CSF leakage is very rare in children because the skull is flexible and the sinuses are underdeveloped [9,20].
The development of CSF rhinorrhea is possible through the frontal, ethmoid, and sphenoid sinus pathways. The frontal and ethmoid sinuses appear to be the most common sites for CSF leakage, especially the ethmoid sinus, which is very thin and can easily fracture even with minor TBI. In these cases, the CSF fistula opens into the middle ear canal or eustachian tube from the sphenoid sinus or pyramidal bone through the frontal sinus, filamentous plate, and sella turcica.
CSF leakage occurs within 48 hours after trauma in 55% of cases and in 70% within 1 week following trauma; however, in 10–20% of cases, it occurs several years after trauma, and in rare cases, it occurs 10 years later [9]. In most cases, CSF leakage due to trauma is spontaneous and improves in 20–30% of patients within 1 week, in 70% of patients within a few months, and in most patients within 6 months [16].
The mechanism of spontaneous CSF leakage may be caused by the attachment of blood or inflammatory by-products to the associated skull fracture site or dura mater, or by the escape of brain tissue to the traumatic defect site. In fact, even if there is post-traumatic CSF leakage, it often resolves without notice, and in many cases, it stops spontaneously in the early stage. CSF leakage is especially difficult to diagnose in infants and young children. There may be no complications even if there is a continuous CSF leak, but postural headache and pneumocephalus may occur.
In the diagnosis of traumatic CSF leakage, the presence of otorrhea and rhinorrhea should be examined, and the region of the skull defect should be determined. The most common method to detect CSF leakage is glucose oxidase test strips; however, these tests have low sensitivity and specificity and are therefore not completely reliable. β2-transferrin and β-trace protein in otorrhea and rhinorrhea, respectively, have recently emerged as highly sensitive and specific methods for detecting CSF leakage.
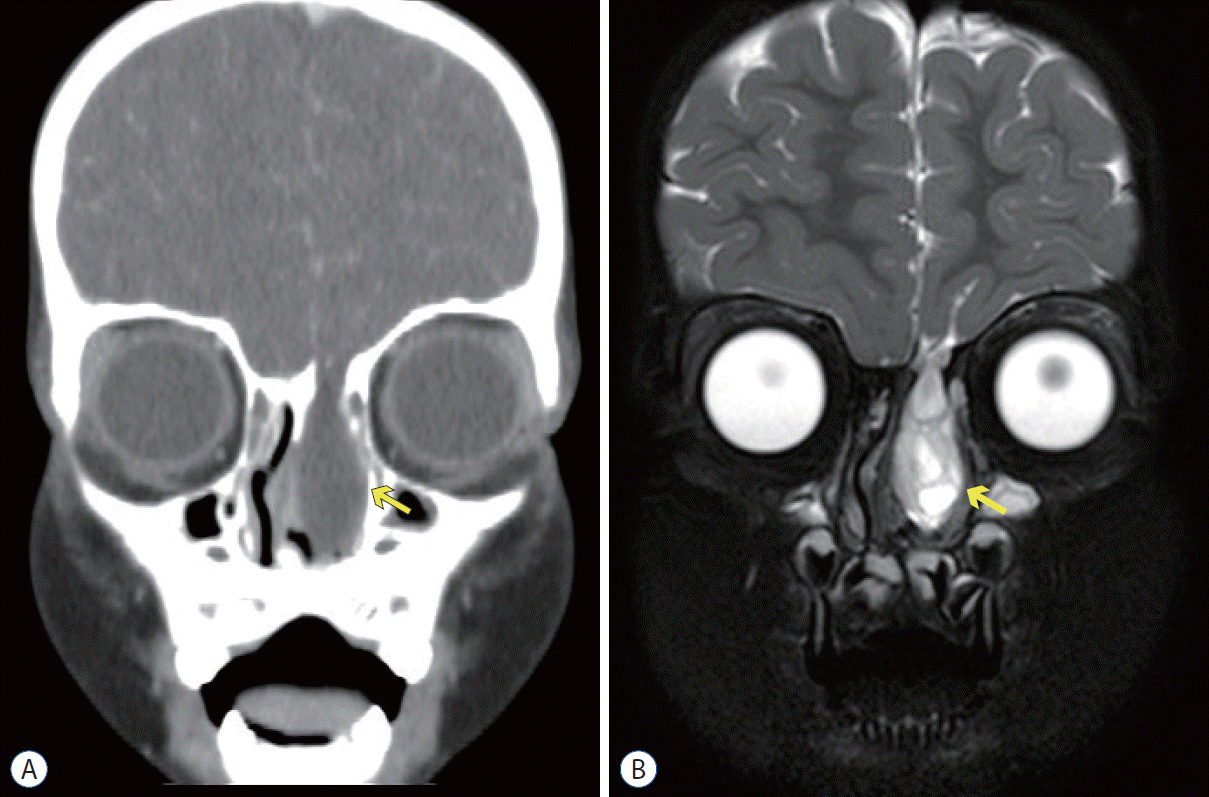
High-resolution CT (HRCT) is the most useful radiologic method for diagnosing a CSF fistula and predicting the likelihood of spontaneous healing. HRCT uses 1- to 2-mm sections in both the axial, coronal, and sagittal planes, resulting in localization of the basal skull fractures that result in CSF leakage.
MRI is another useful radiologic test to detect CSF rhinorrhea, especially in cases of inflammatory sinus disease. It may help differentiate between mucopurulent discharge and CSF, whereas they may show the same radiopacity on CT (Fig. 2) [9]. If there is a CSF fistula or a bony defect after head trauma, abnormalities may not be found on HRCT or MRI. In this case, it can be confirmed using intrathecal fluorescein or radionuclide cisternography [28].
Meningitis is the most serious complication of CSF leakage and is known to occur in approximately 24% of untreated CSF rhinorrhea cases. The causative agents of meningitis associated with pediatric traumatic CSF leakage include Streptococcus pneumonia, Staphylococcus aureus, Enterobacter spp., Escherichia coli, and Klebsiella pneumonia, among which Streptococcus pneumonia is known to be the most common. However, recent research has shown that gram-negative bacilli are the main causative bacteria, and most of them are known to be vulnerable to prophylactic antibiotic therapy [3,21].
For the treatment of pediatric traumatic CSF leakage, although controversy remains, some argue that surgical correction of the fistula is necessary to prevent recurrence of bacterial meningitis and continuous leakage of CSF.
Multiple surgical approaches can be used for treatment of pediatric traumatic CSF leakage.
An intracranial approach through bi-frontal craniotomy with dural repair is mainly used for anterior fossa CSF leakage. In cases of CSF rhinorrhea due to ethmoidal fractures, external ethmoidectomy with obliteration of the dural defect with muscle or fat packing is appropriate. In these cases, the lateral rhinotomy approach has been generally used, and good surgical outcomes have recently been reported through the endonasal endoscopic approach. Sub-temporal craniotomy with primary dural closure can be used for repair of CSF leakage in the middle cranial fossa.
In some cases, conservative treatments such as stabilization, head elevation, and movement restriction are performed until the leakage stops spontaneously [32].
However, even if CSF leakage spontaneously improves, there are cases where the defect of the dura mater remains, causing repeated rhinorrhea and the risk of bacterial meningitis [2]. In addition, in the case of CSF leakage, prophylactic antibiotic therapy is sometimes implemented to prevent recurrence of bacterial meningitis, but its effectiveness remains controversial [2,32].
Traumatic CSF leakage in pediatric patients is a rare complication of TBI, but it carries significant morbidity and mortality if not promptly diagnosed and managed. Conservative treatment should be initiated immediately, and surgical intervention should be considered if central nervous system infections such as meningitis and intracranial abscess due to persistent CSF leakage are suspected.
Mild TBI and concussion are often used synonymously, with no universally agreed upon definition of either. Concussion was recently defined by the American Academy of Neurology as “a clinical syndrome of biomechanically induced alteration of brain function that is trauma-induced and involves an alteration in mental status that may or may not include loss of consciousness” [12]. Concussions can occur when acceleration-deceleration and/or rotational forces are applied to the head.
Because children’s heads are relatively larger and heavier than those of adults, and their skulls are more compliant, pediatric patients are more susceptible to TBI [27]. Children also have weaker neck muscles and, most importantly, their neurons are less myelinated than those of adults. Therefore, the pediatric brain is not only more likely to sustain greater shearing forces when a force is applied but also more prone to axonal damage because of decreased myelination [17,27].
The diagnosis of concussion in children is mainly based on a combination of symptoms after TBI. Various symptoms such as headache, fatigue, irritability, anxiety, photophobia, dizziness, amnesia, and disorientation can be caused by pediatric concussion [6]. Conventional neuroimaging (CT and MRI) findings are typically normal in pediatric concussion and are used to determine severe brain injury and edema that require emergent neurosurgical intervention. However, the usefulness of CT and MRI in diagnosing concussion in pediatric patients is not known when neurosurgical intervention is not deemed necessary. Several advanced neuroimaging techniques, including functional MRI and diffusion tensor imaging, may have the potential to increase the sensitivity of neuroimaging to detect both structural and functional abnormalities associated with pediatric concussion [5]. Recently, various approaches such as serum biomarkers, balance tests, and cognitive tests have been used to diagnose pediatric concussion [22,26].
Although the treatment of pediatric concussion is controversial, most studies have reported that immediate removal from play after concussion helps with recovery [8]. Second impact syndrome without adequate recovery in pediatric patients with concussion is rare but can lead to severe brain injury [25,30].
The appropriate rest period after pediatric concussion is unknown. Studies of the pathophysiology of pediatric concussion have shown that metabolic changes after a concussion can last from several days to weeks [11]. However, current evidence suggests that prolonged periods of rest are associated with prolonged recovery times. The most recent consensus statement from the International Consensus on Concussion in Sports recommends a brief period of rest lasting 24–48 hours, followed by a gradual and progressive return [25].
The International Classification of Diseases-10 coding system states that organic disturbances (headache, dizziness, and sleep problems) and psychogenic disturbances (irritability, emotional issues, and affect changes) after closed head injuries that are chronic, permanent, or late-emerging may be termed post-concussion syndrome. Although most pediatric concussion patients recover within a few weeks of TBI, some have been reported to experience post-concussion symptoms. Although the pathophysiology of post-concussion is still unknown, various factors, including the type and extent of TBI, premorbid factors, vestibular symptoms, number of prior concussions, and initial symptoms may contribute to delayed resolution after pediatric TBI [4]. The need for multidisciplinary treatment has recently emerged [15]. This includes vestibular therapy, psychological counseling, cognitive rehabilitation, and headache management.
Pediatric minor TBI remains a public health concern, with complex interactions between injury and the ongoing development of the immature brain. In this review article, the authors reviewed the existing literature on pediatric minor TBIs, particularly GSF, post-traumatic CSF leakage, and concussion.
Notes
References
1. Abuzayed B, Tuzgen S, Canbaz B, Yuksel O, Tutunculer B, Sanus GZ. Reconstruction of growing skull fracture with in situ galeal graft duraplasty and porous polyethylene sheet. J Craniofac Surg. 20:1245–1249. 2009.

2. Bell RB, Dierks EJ, Homer L, Potter BE. Management of cerebrospinal fluid leak associated with craniomaxillofacial trauma. J Oral Maxillofac Surg. 62:676–684. 2004.

3. Bernal-Sprekelsen M, Bleda-Vázquez C, Carrau RL. Ascending meningitis secondary to traumatic cerebrospinal fluid leaks. Am J Rhinol. 14:257–259. 2000.

4. Bernard CO, Ponsford JA, McKinlay A, McKenzie D, Krieser D. Predictors of post-concussive symptoms in young children: injury versus non-injury related factors. J Int Neuropsychol Soc. 22:793–803. 2016.

7. de Djientcheu VP, Njamnshi AK, Ongolo-Zogo P, Kobela M, Rilliet B, Essomba A, et al. Growing skull fractures. Childs Nerv Syst. 22:721–725. 2006.

8. Elbin RJ, Sufrinko A, Schatz P, French J, Henry L, Burkhart S, et al. Removal from play after concussion and recovery time. Pediatrics. 138:e20160910. 2016.

9. Eljamel MS, Foy PM. Acute traumatic CSF fistulae: the risk of intracranial infection. Br J Neurosurg. 4:381–385. 1990.

10. Ellis TS, Vezina LG, Donahue DJ. Acute identification of cranial burst fracture: comparison between CT and MR imaging findings. AJNR Am J Neuroradiol. 21:795–801. 2000.
11. Giza CC, Hovda DA. The new neurometabolic cascade of concussion. Neurosurgery 75 Suppl. 4:S24–S33. 2014.

12. Giza CC, Kutcher JS, Ashwal S, Barth J, Getchius TS, Gioia GA, et al. Summary of evidence-based guideline update: evaluation and management of concussion in sports: report of the guideline development subcommittee of the American Academy of Neurology. Neurology. 80:2250–2257. 2013.

13. Hussain E. Traumatic brain injury in the pediatric intensive care unit. Pediatr Ann. 47:e274–e279. 2018.

14. Husson B, Pariente D, Tammam S, Zerah M. The value of MRI in the early diagnosis of growing skull fracture. Pediatr Radiol. 26:744–747. 1996.

15. Janak JC, Cooper DB, Bowles AO, Alamgir AH, Cooper SP, Gabriel KP, et al. Completion of multidisciplinary treatment for persistent postconcussive symptoms is associated with reduced symptom burden. J Head Trauma Rehabil. 32:1–15. 2017.

16. Kendirli T, Unay B, Tosun F, Hacihamdioğlu B, Akin R, Ozkaptan Y, et al. Recurrent Streptococcus pneumoniae meningitis in a child with traumatic anterior cranial base defect. Pediatr Int. 48:91–93. 2006.

17. Kimbler DE, Murphy M, Dhandapani KM. Concussion and the adolescent athlete. J Neurosci Nurs. 43:286–290. 2011.

18. Kutlay M, Demircan N, Akin ON, Basekim C. Untreated growing cranial fractures detected in late stage. Neurosurgery. 43:72–76. discussion 76-77. 1998.

19. Leung GK, Chan KH, Hung KN. Growing skull fracture in an adult nine years after blunt head trauma. J Clin Neurosci. 18:855–857. 2011.

20. Mantur M, Łukaszewicz-Zając M, Mroczko B, Kułakowska A, Ganslandt O, Kemona H, et al. Cerebrospinal fluid leakage--reliable diagnostic methods. Clin Chim Acta. 412:837–840. 2011.

21. Matschke J, Tsokos M. Post-traumatic meningitis: histomorphological findings, postmortem microbiology and forensic implications. Forensic Sci Int. 115:199–205. 2001.

22. McCarthy MT, Kosofsky BE. Clinical features and biomarkers of concussion and mild traumatic brain injury in pediatric patients. Ann N Y Acad Sci. 1345:89–98. 2015.

23. Jamjoom Z, Jamjoom A, Murshid WR. Growing skull fractures: classification and management. Br J Neurosurg. 8:667–679. 1994.

24. Nalls G, Lightfoot J, Lee A, Blackwell L. Leptomeningeal cyst: nonenhanced and enhanced computed tomography findings. Am J Emerg Med. 8:34–35. 1990.

25. Pfaller AY, Nelson LD, Apps JN, Walter KD, McCrea MA. Frequency and outcomes of a symptom-free waiting period after sport-related concussion. Am J Sports Med. 44:2941–2946. 2016.

26. Pickering A, Harnan S, Fitzgerald P, Pandor A, Goodacre S. Clinical decision rules for children with minor head injury: a systematic review. Arch Dis Child. 96:414–421. 2011.

27. Pinto PS, Poretti A, Meoded A, Tekes A, Huisman TA. The unique features of traumatic brain injury in children. Review of the characteristics of the pediatric skull and brain, mechanisms of trauma, patterns of injury, complications and their imaging findings--part 1. J Neuroimaging. 22:e1–e17. 2012.

28. Rocchi G, Caroli E, Belli E, Salvati M, Cimatti M, Delfini R. Severe craniofacial fractures with frontobasal involvement and cerebrospinal fluid fistula: indications for surgical repair. Surg Neurol. 63:559–563. discussion 563-564. 2005.

29. Singh I, Rohilla S, Siddiqui SA, Kumar P. Growing skull fractures: guidelines for early diagnosis and surgical management. Childs Nerv Syst. 32:1117–1122. 2016.

30. Upchurch C, Morgan CD, Umfress A, Yang G, Riederer MF. Discharge instructions for youth sports-related concussions in the emergency department, 2004 to 2012. Clin J Sport Med. 25:297–299. 2015.

31. Vezina N, Al-Halabi B, Shash H, Dudley RR, Gilardino MS. A review of techniques used in the management of growing skull fractures. J Craniofac Surg. 28:604–609. 2017.

32. Yilmazlar S, Arslan E, Kocaeli H, Dogan S, Aksoy K, Korfali E, et al. Cerebrospinal fluid leakage complicating skull base fractures: analysis of 81 cases. Neurosurg Rev. 29:64–71. 2006.

33. Yoshioka H, Sakoda K, Kohno H, Hada H, Kurisu K. Usefulness of color Doppler sonography in a growing skull fracture: case report. J Trauma. 42:144–146. 1997.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download