This article has been retracted. See "Extracranial Carotid-Vertebral Artery Bypass Technique and Surgical Outcomes" in Volume 67 on page 257.
Abstract
Vertebral artery (VA) occlusion is frequently encountered, usually without acute ischemic injury of the brain. However, when it is accompanied by hypoplasia or stenosis of the opposite VA, brain ischemia may develop due to insufficient collateral supply. Both hemodynamic instability and embolic infarction can occur in VA occlusion, which may cause severe symptoms in a patient. Extracranial carotid-VA bypass should be considered for symptomatic VA occlusion patients, especially when the patient has repeated ischemic brain injuries. In this report, the cases of three extracranial carotid-VA bypass patients are introduced, along with a brief description of the surgical techniques. All three cases were treated with different bypass methods according to their disease location.
Posterior circulation strokes are reported to have 20% to 30% higher mortality rates than anterior circulation strokes [7,8]. Although the mortality rate of posterior circulation strokes is higher, diagnosis is often difficult due to its subtle initial symptoms. The symptoms of vertebrobasilar ischemia (VBI) include dizziness, vertigo, drop attacks, diplopia, perioral numbness, alternating paresthesia, tinnitus, dysphasia, dysarthria, and ataxia [6]. The pathogenesis of VBI can be either hemodynamic or embolic, however, emboli occur more frequently in symptomatic patients and are more dangerous and fatal [9]. The treatment of posterior circulation stroke is similar to that of anterior circulation stroke, which includes the use of antiplatelet or anticoagulant therapy. Surgical vertebral artery (VA) reconstruction may be considered for severe patients with 1) both VA stenosis of more than 60% with hemodynamic symptoms, 2) occlusion of the VAs with opposite VA hypoplasia, 3) symptomatic VBI due to emboli or 4) occlusion of the carotid arteries causing global ischemia [1,9]. Surgical VA reconstruction includes vertebrocarotid transposition or graft interposition [5]. Surgeons are often pessimistic about VA reconstruction due to the high level of technical difficulty required and the perioperative complications associated with the procedure. Here, we report three cases of successful extracranial carotid-VA bypasses to emphasize the importance of surgical reconstruction in improving patient’s symptoms and long-term morbidity.
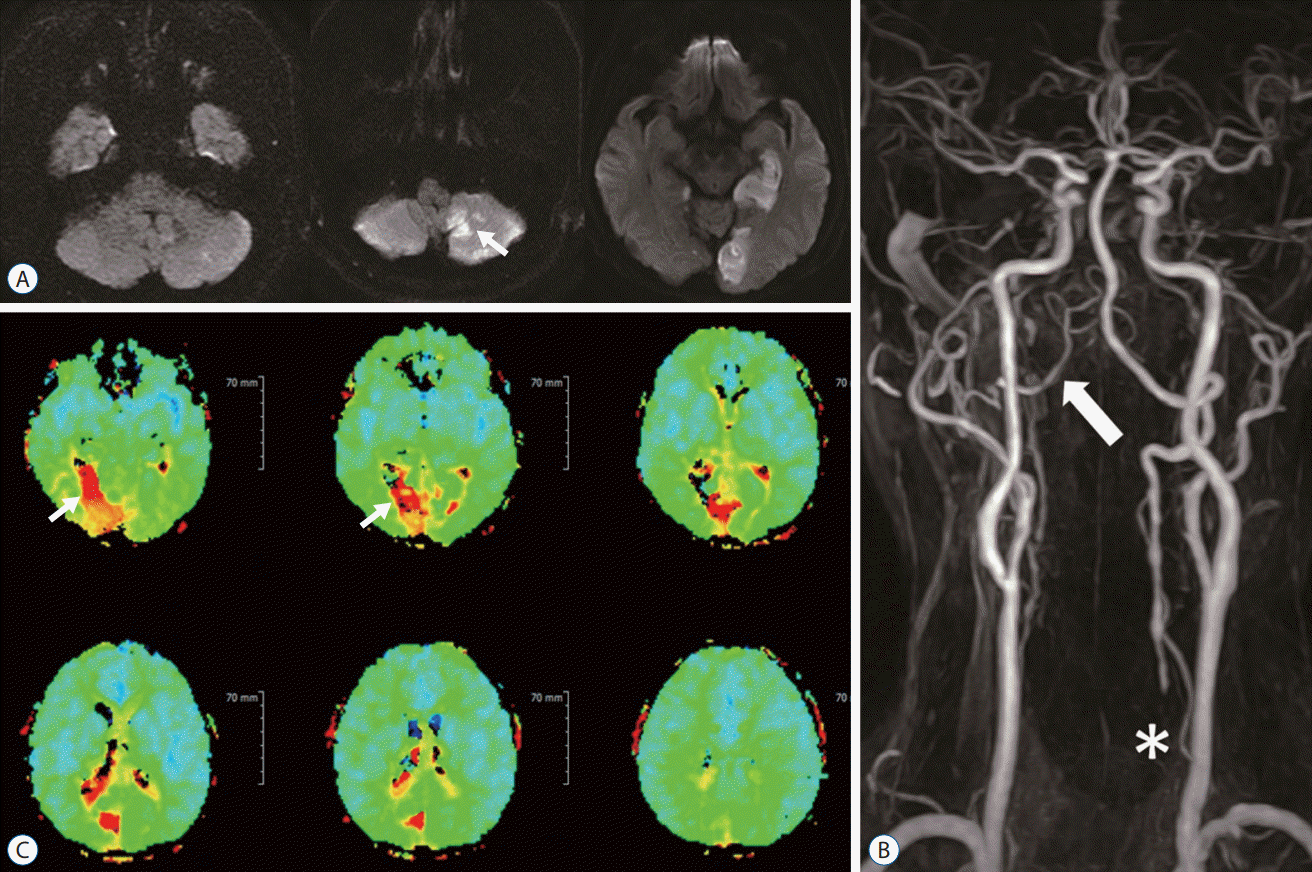
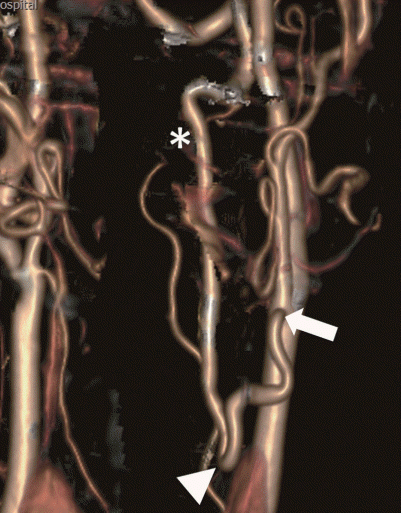
A 63-year-old male with a history of diabetes mellitus and smoking developed a sudden onset of gait disturbance and right homonymous hemianopsia. The patient suffered from intermittent dizziness for 5 years but did not have a previous brain evaluation. As soon as the patient was admitted to Bucheon Saint Mary's Hospital, brain magnetic resonance imaging (MRI) was conducted, which displayed right cerebellum and left occipital infarctions on diffusion-weighted imaging (Fig. 1A). For further evaluation, a magnetic resonance angiogram (MRA) and perfusion MRI were performed. The MRA demonstrated right VA hypoplasia, left proximal VA occlusion, and left posterior cerebral artery (PCA) occlusion (Fig. 1B). A hemodynamic study with perfusion MRI revealed sufficient blood flow through the collateral channel networked by muscular branches. After confirmation of the diagnosis by digital subtraction angiography (DSA), the patient was treated with low-molecular-weight heparin for 2 weeks followed by 100 mg aspirin and 75 mg clopidogrel. However, medical therapy was not sufficient, and the patient had second and third embolic infarctions in the cerebellum within 2 months. Transposition of the VA from the V2 segment (VA segment passing through the transverse foramen of cervical vertebrae C6 to C2) to the common carotid artery (CCA) was performed and the patient recovered without any neurological deficits (Fig. 2).
The procedure was performed with an 8 cm linear incision approximately 3 cm above and parallel to the clavicle. After dissection of the platysma and sternocleidomastoid muscle, the CCA was isolated by retracting the jugular vein laterally. Foraminotomy of the C5 transverse foramen allowed for visualization of the VA. The VA was then ligated at the proximal and distal site with hemoclips and divided to obtain a free end for anastomosis. It is important to obtain a sufficient length of VA for transposition to the CCA. Next, arterial clamps were applied at the proximal and distal end of the CCA for arteriotomy on the lateral wall of the CCA, and an end-to-side anastomosis with the VA was performed. A brief release of the arterial clamp before the final suture, irrigation with heparin, then finally releasing the arterial clamps in the order of distal VA-proximal CCA-distal CCA, were all necessary steps to prevent thrombus formation and embolic infarctions.
CCA-VA bypass was performed without artery or vein graft because the CCA and VA were located in close proximity to each other. Moreover, the proximal part of VA was incised after ligation to make available for direct anastomosis.
A 63-year-old male was admitted with repeated transient left hemiplegia. MRI, MRA, perfusion MRI, and DSA revealed right CCA occlusion, causing hemodynamic insufficiency on the right hemisphere (Fig. 3). Additionally, there was severe stenosis in the left proximal internal carotid artery (ICA) and right V4 segment (intracranial segment of the VA), limiting the collateral supply to the right hemisphere. The proatlantal intersegmental artery collateral to the CCA was not enough, causing transient ischemic attack symptoms. Considering the above results, we performed a CCA to V2 graft bypass using a radial artery (RA) graft. The patient recovered without any significant complications, and perfusion MRI displayed hemodynamic improvements (Fig. 4).
The surgical technique used was similar to the procedure mentioned in case 1, except that we used an RA graft, which was harvested by a cardiovascular surgeon. For this patient, the C5 transverse process was extracted to expose the VA, and the RA graft to the VA anastomosis was performed first. The proximal ICA, CCA, and proximal external carotid artery (ECA) were occluded temporarily in sequence with a vessel punch for arteriotomy of the CCA, followed by end-to-side anastomosis with the RA graft. Completing the VA-RA first before clamping the CCA, reduced the risk of cerebral ischemic injury and thrombus formation.
CCA-RA-VA bypass was performed to restore hemodynamic stability of the right hemisphere. RA graft was used for anastomosis because the VA could not be ligated for transposition. In comparison to the saphenous vein graft, RA graft is less direction sensitive for not having venous valves and is associated with higher patency rate.
A 58-year-old male was found to have multiple posterior infarctions after presenting to our hospital with double hemiplegia, dysarthria, dysphagia, and cognitive dysfunction. The patient had a history of diabetes mellitus, hypertension, and end-stage renal disease. He was diagnosed with left cerebellum and right thalamus infarction 1 year prior to the recurrence of acute infarction in the same region (Fig. 5A). Before admission to our hospital, consecutive medical treatment with anticoagulants and 30 mg rivaroxaban was used, and his underlying diabetes and hypertension were well controlled. MRA and perfusion MRI revealed right VA hypoplasia with left proximal VA occlusion, along with time-to-peak prolongation on the right PCA territory (Fig. 5B and C). To prevent progression and recurrence of the infarction, we performed an ECA to V2 graft bypass, using a saphenous vein graft (Fig. 6). The patient improved neurologically and was able to swallow.
During the surgery, a similar technique to those mentioned above was performed to release the VA from the C5 transverse foramen. However, for this patient, we harvested the saphenous vein graft for the bypass. To minimize rotation and malpositioning of the vein, we first made a line on the surface of the vein with a marking pen. In the process of the VA-saphenous vein graft-ECA anastomosis, an atheroma on the VA was observed and resected. Additionally, there was a partial arterial wall dissection of the VA, which was repaired with doublearm needle sutures. Patency of the anastomosis was checked with indocyanine green videoangiography and an intraoperative angiogram.
ECA-saphenous vein-VA bypass was performed to reconstitute flow to the posterior circulation. Saphenous vein graft has the advantage of being longer than the RA, which was critical for anastomosis between proximal VA and ECA.
Progression of VBI with severe stenosis or occlusion of the VAs can lead to devastating results when left untreated. Medical treatment may resolve the problem in many patients but some patients, especially those with repeated ischemic attacks, should be treated surgically because they have a 30–35% stroke risk over a 5-year period [5]. In a previous study, 69% of patients was reported to achieve complete resolution of VBI symptoms after intracranial-to-extracranial anastomosis [11]. However, patients with large- and medium-sized vessel occlusions with poor collaterals may not be adequate for intracranial-to-extracranial anastomosis. Such patient may benefit more from the extracranial carotid-to-VA bypass as shown in our cases.
Surgical VA reconstruction includes vertebrocarotid transposition and graft interposition. Studies suggest that the outcome of bypass reconstruction differs depending on the location of the bypass site. Although there are advantages and disadvantages of both proximal and distal reconstructions, proximal reconstruction is reported to have combined death and stroke rates of 0.9% compared to 3% for distal reconstructions [4]. Predictably, we had no occurrence of stroke or death in any of our extracranial carotid-VA bypass patients, all performed with proximal reconstruction. Perioperative complications for proximal VA reconstruction include immediate thrombosis (1.4%), vagus and recurrent laryngeal nerve palsy (2%), Horner syndrome (8.4–28%), lymphoceles (4%), and chylothorax (5%), and, rarely, stroke or hematomas [1-3,10].
It is important to correctly locate and prepare the bypass end on both the donor and recipient vessels. When the harvested VA is too short, traction and dissection can occur, whereas torsion and coagulation can be induced when it is too long. Furthermore, adequate dissection of the anastomosis site is recommended to be able to visualize each suture clearly and to reduce the entire operative time [1,5,9]. Bypass operations performed with our technique were completed within 180 minutes in all three cases presented in this report.
Compared to the anterior circulation strokes, the symptoms of posterior circulation strokes can disappear when treated correctly. Therefore, surgeons should evaluate such patient thoroughly, and provide a surgical reconstruction by extracranial carotid-VA bypass when indicated.
Notes
Informed consent
Informed consent was obtained from all individual participants included in this study.
References
1. Berguer R. Mechanical compression of the vertebral arteries. In : Berguer R, Bauer RB, editors. Vertebrobasilar arterial occlusive disease: Medical and surgical management. New York: Raven Press;1984. p. 45–71.
2. Berguer R. Distal vertebral artery bypass: technique, the "occipital connection," and potential uses. J Vasc Surg. 2:621–626. 1985.

4. Berguer R. Complex Carotid and Vertebral Revascularizations. In : Pearce WH, Matsumura JS, Yao JST, editors. Vascular Surgery in the Endovascular Era. Evanston: Greenwood Academic;2008. p. 344–352.
5. Berguer R, Morasch MD, Kline RA. A review of 100 consecutive reconstructions of the distal vertebral artery for embolic and hemodynamic disease. J Vasc Surg. 27:852–859. 1998.

6. Caplan LR, Wityk RJ, Glass TA, Tapia J, Pazdera L, Chang HM, et al. New england medical center posterior circulation registry. Ann Neurol. 56:389–398. 2004.

7. Chastain HD 2nd, Campbell MS, Iyer S, Roubin GS, Vitek J, Mathur A, et al. Extracranial vertebral artery stent placement: in-hospital and follow-up results. J Neurosurg. 91:547–552. 1999.

8. Kieffer E, Praquin B, Chiche L, Koskas F, Bahnini A. Distal vertebral artery reconstruction: long-term outcome. J Vasc Surg. 36:549–554. 2002.

9. Kieffer E, Rancurel G, Richard T. Reconstruction of the distal cervical vertebral artery. In : Berguer R, Bauer RB, editors. Vertebrobasilar arterial occlusive disease: Medical and surgical management. New York: Raven Press;1984. p. 265–289.
Fig. 1.
A : Images of diffusion-weighted brain magnetic resonance imaging show multiple infarctions in the right cerebellum and the left occipital lobe (white arrows). B : Digital subtraction angiogram of left vertebral artery revealed an occlusion of vertebral artery origin (white arrow) and retrograde filling of vertebral artery (V2 segment, asterisk) through cervical muscular branch of subclavian artery (black arrow).
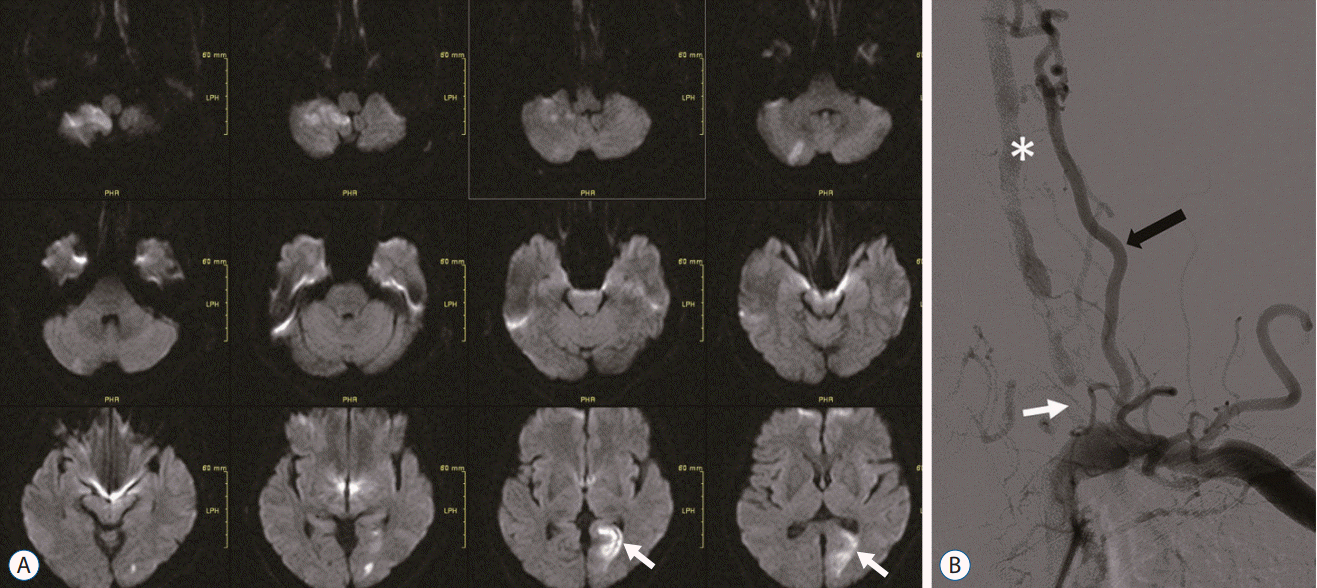
Fig. 2.
A : Postoperative computerized tomography angiography volume rendering image after surgery show transposition of the proximal vertebral artery (white arrow) to the common carotid artery (white asterisk) with clipping of proximal vertebral artery origin (white arrowhead). B : Post operative digital subtraction angiogram with 3 dimensional reconstruction image show end to side bypass of vertebral artery to common carotid artery with good patency.
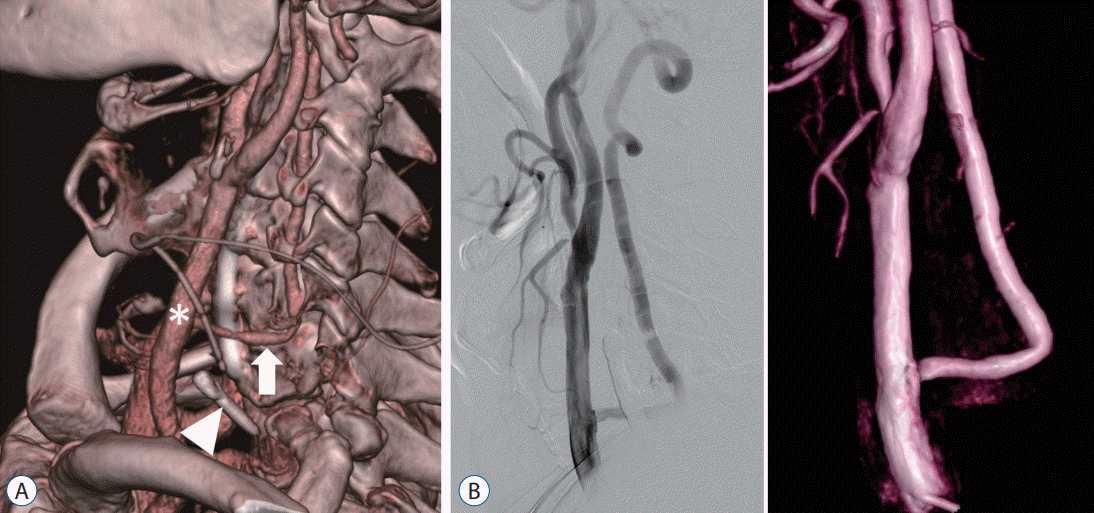
Fig. 3.
A : Magnetic resonance angiogram reveal right common carotid artery occlusion (white asterisk) with left proximal internal carotid artery (white arrow) and 4th segment of right vertebral artery stenosis. B : Time-to-peak image of the perfusion magnetic resonance imaging show hemodynamic insufficiency in the right hemisphere.
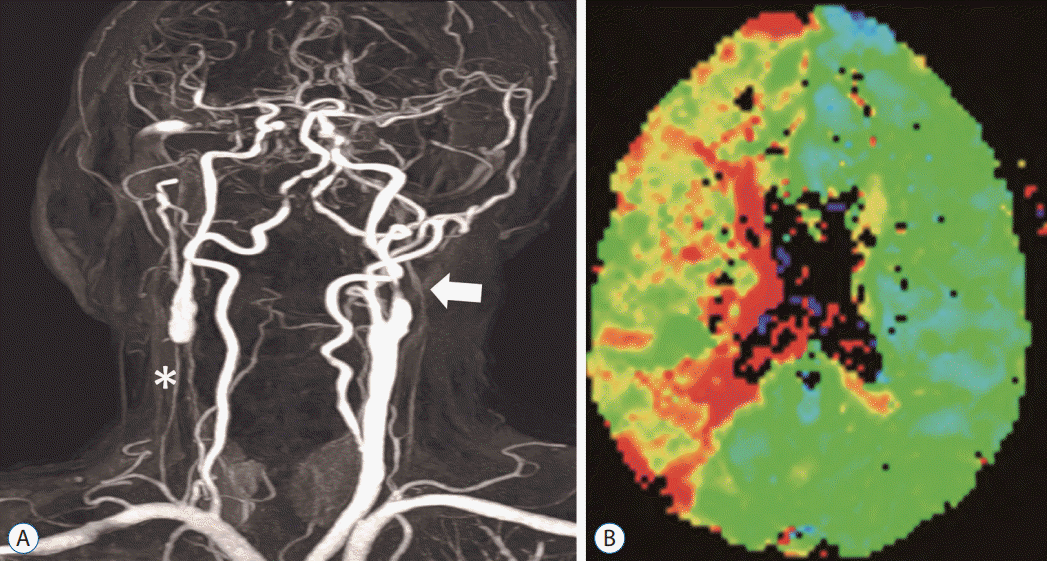
Fig. 4.
A : Postoperative computerized tomography angiography volume rendering image after bypass surgery show right common carotid artery (arrow) is connected with right vertebral artery (arrowhead) by the graft vessel (asterisk). B : Postoperative digital subtraction angiography with right vertebral artery (arrowhead) selection show blood flow from the vertebral artery to the anterior circulation via common carotid artery. C : Postoperative time-to-peak image of perfusion magnetic resonance display hemodynamic improvements on right hemisphere (asterisk).
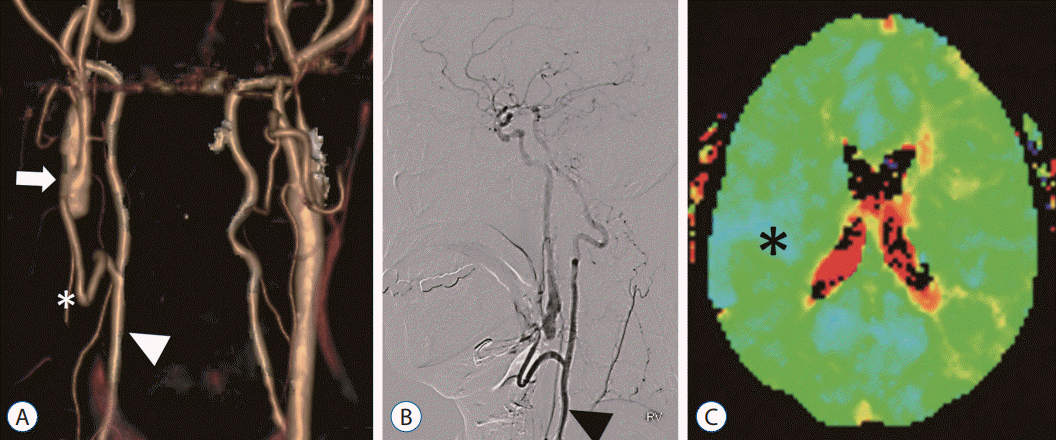
Fig. 5.
A : Image of diffusion-weighted brain magnetic resonance imaging shows infarction in the right cerebellum (white arrow). B : Magnetic resonance angiogram revealed right vertebral artery hypoplasia (arrows) with left proximal vertebral artery occlusion (asterisk). C : Perfusion magnetic resonance imaging shows time-to-peak prolongation on the right posterior cerebral artery territory (white arrows).