Abstract
Objective
Extracranial supra-aortic dissections (ESADs) with severe stenosis, occlusion and/or pseudoaneurysm presents potential risk of stroke. Endovascular stenting to reconstruct non acute phase ESADs (NAP-ESADs) is an alternative to anticoagulant or antiplatelet therapy. However, its feasibility, safety and efficacy of stenting in NAP-ESADs is unclear. This study aims to investigate the long-term outcomes of the feasibility, safety and efficacy of stenting in NAP-ESADs.
Methods
Seventy-four patients with 91 NAP-ESAD vessels with severe stenosis, occlusion and/or pseudoaneurysm presents potential risk of stroke who underwent stent remodeling were enrolled into this respective study from December 2008 to March 2020. Technical success rate, complications, clinical and angiographic results were harvested and analyzed.
Results
Success rate of stent deployment was 99% (90/91) with no procedural mortality or morbidity. Transient ischemic attack occurred in three patients during operation (4.1%, 3/74). Asymptomatic embolisms of distal intracranial vessels were found in two patients (2.7%, 2/74). One hundred and forty-two stents deployed at 85 carotid (135 stents) and six vertebral (seven stents) vessels. Six stent types (Wingspan, 28/135, 20.7%; Solitaire, 10/135, 7.4%; Neuroform, 8/135, 5.9%; LVIS, 2/135, 1.5%; Precise, 75/135, 55.6%; Acculink, 12/135, 8.9%) were deployed at carotid arterial dissection while two types (Wingspan, 5/7, 71.4%; Solitaire 2/7, 28.6%) at vertebral arterial dissection. Digital subtracted angiography (56%, 51/91), computational tomography angiography (41.8%, 38/91) and high resolution magnetic resonance imaging (2.2%, 2/91) were adopted for follow up, with a mean time of 17.2±15.4 months (5–77). All patient modified Rankin Scale scores showed no increase at discharge or follow-up. Angiographically, dissections in 86 vessels in 69 patients (94.5%, 86/91) were completely reconstructed with only minor remnant dissections in four vessels in four patients (4.4%, 4/91). Severe re-stenosis in the stented segment required re-stenting in one patient (1.1%, 1/91).
Extracranial supra-aortic dissections (ESADs) mainly include carotid arterial dissection (CAD) and vertebral arterial dissection (VAD) estimated at 3–4 per 100000 [15], which are generally accepted as the etiology of cerebral ischemia, particularly in younger patients (<45 years old) accounting for up to 25% of all ischemic strokes [2,9]. Prior study has shown 4.14% and 1.6% readmission due to ischemic stroke recurrence in patients with symptomatic carotid and vertebral dissections respectively [11]. Endovascular stenting treatment for patients with ESAD is a potentially more effective option than standalone antiplatelet or anticoagulants. As only non-randomized clinical trials of endovascular treatment have been performed for patients with ESADs [14], the feasibility, efficacy and safety of endovascular treatment for ESADs with long-term outcomes is still uncertain. Numerous etiologies were reported including traumatic injury and iatrogenic factors. Primary indications for endovascular stenting of ESADs include persistent potential rupture or thrombotic risk related to acute infarction; secondarily, medical therapy contraindications, contralateral vessel involvement, ongoing distal thromboembolism concerns, and clinically significant compromise of cerebral circulation [12]. This study mainly enrolled high-stenotic, occlusive or pseudoaneurysmal lesions.
The feasibility, safety and efficacy of endovascular stent remodeling of ESADs were investigated in this present study over the past 12 years in our center.
This study was approved and waived the requirement for informed consent by the Institutional Review Board (IRB) of Huashan Hospital Affiliated to Fudan University (IRB No. KY2019-009). Patients who underwent endovascular treatment for ESAD were enrolled in this respective study in our center between December 2008 and March 2020, including those receiving stent deployment or stent assisted embolization. Indications for the treatment of ESAD included high-grade stenosis (>70%), occluded ESAD and/or a dissecting aneurysm. Demographics, medical history, angiographic details, complications and follow up outcomes were extracted. Dissection etiologies were classified into spontaneous, traumatic, or iatrogenic (intraoperational). Dissections were mainly defined as the presence of pseudoaneurysm, beaded expansion, dual lumen sign, along with stenosis or occlusion from floating intimal flap according to different imaging modalities (computed tomography angiography [CTA], magnetic resonance angiography, high resolution magnetic resonance imaging [HR-MRI], digital subtracted angiography [DSA]) and MRI for intermural hematoma. The location of vessel dissection was divided into common carotid artery (CCA), extracranial internal carotid artery (ICA), and vertebral artery (VA).
Stent selection varied according to dissection location. For CAD lesions in the CCA or cervical segment of the ICA, an Acculink stent (Abbott Vascular, Santa Clara, CA, USA) or Precise stent (Cordis, Miami Lakes, FL, USA) were preferred. For petrosal and cavernous segments of the ICA or VAD segment, a number of different stents were viable (Neuroform3, Wingspan, LVIS [low-profile visualized intraluminal support device], and Solitaire). Generally, distal protection device filters were rarely used to avoid embolism, except for some multiple lumen or high-volume thrombus dissection segments utilized. For tortuous dissections in the ICA cervical segment, guiding-catheter-assisted stenting was recommended.
Procedurally : 1) the guiding catheter is navigated across the dissecting segment supported by a 0.35 wire or dual wire, or a Gateway balloon (Boston Scientific Inc., Marlborough, MA, USA); 2) the stent delivery system is positioned; 3) retrieval of the guiding catheter; and 4) stent deployment to repair any torn intima and straighten the tortuous dissection segment (Fig. 1). Navigating the guiding catheter across the tortuous dissection segment is intended to increase stent accessibility. If a stent could not be fully expanded for dissections with intermural thrombus and/or hematoma, then balloon angioplasty should be performed.
General heparinization is performed through a bolus injection of heparin 1250 international units (IU) per 15 kg before procedure. An additional half dose of previous bolus of heparin every hour until at least 1250 IU/h was injected throughout the procedure. For the high-grade stenosis (>70%) or occluded ESAD, postoperative systolic blood pressure (generally 90–120 mmHg) should be monitored strictly to avoid hyper-perfusion syndrome or hemorrhage. The non-acute phase of ESAD was defined as after 8 hours from symptom onset [3]. Acute phase ESAD cases have high basal National Institutes of Health Stroke Scale (NIHSS) score, more unilateral lesions and relatively poor prognosis. In this study, acute phase ESAD cases were excluded. Dual antiplatelet therapy (aspirin, 100 mg/d; clopidogrel, 75 mg/d) routinely administered preoperatively for 3 days. Patients without antiplatelet preparation before operation should receive intravenous Tirofiban (0.01 mg/kg; >24 hours) bridging with dual antiplatelets post-stenting. Follow up was scheduled at postoperative 6–12 months using CTA, DSA or HR-MRI.
Frequencies (percentages [ranges] and means±standard deviations] are used to describe the CAD and VAD cohorts. The increased modified Rankin Scale (mRS) and larger than 2 scores were classified into poor outcomes.
Seventy-four patients (53 male, 71.6%) 91 vessels were retrospectively harvested with mean age 54.3±10.2 years old (range, 30–76). Bilateral dissections in 15 patients (15/74, 20.3%) were collected from 83 single carotid dissections in 68 patients (68/74, 91.9%), while four patients of single VA (4/74, 5.4%) and two patients simultaneously involved carotid and VA (2/74, 2.7%).
Thirty-seven acute stroke patients (50.0%) and 13 transient ischemic attack patients (17.4%) in total had persistent symptoms with a mean time of 77.95±131.32 days (0.25–730) after the initial dissection diagnosis. In addition, 24 dissections (32.6%) embodying repeated dizziness, headache or neck pain with a mean time of 88.79±91.00 days (1–365) were found while patients were being treated for other ipsilateral cerebral artery lesions including intracranial aneurysms and arteriovenous malformations. Dissections were caused: spontaneously in 61 patients and 75 vessels, by trauma (n=7/9), from carotid radiotherapy (4/5) and iatrogenically (2/2).
Dissecting segment mainly presented three morphologic changes : 1) pure stenosis (33/91, 36.3%); 2) occlusion (2/91, 2.2%); and 3) dissecting aneurysm with stenosis or non-stenosis (56/91, 61.5%). For the large dissecting aneurysm, stent assisted coiling was adopted (19/56, 33.9%). For dissection with stenosis and/or small dissecting aneurysm, the stent solely was deployed (37/56, 66.1%; Table 1).
One hundred and forty-two stents deployed at 85 carotid (135 stents) and six vertebral (seven stents) vessels. Six stent types (Wingspan, 28/135, 20.7%; Solitaire, 10/135, 7.4%; Neuroform, 8/135, 5.9%; LVIS, 2/135, 1.5%; Precise, 75/135, 55.6%; Acculink, 12/135, 8.9%) were deployed at CAD while two types (Wingspan, 5/7, 71.4%; Solitaire 2/7, 28.6%) at VAD. Total overlapped stents were adopted at five vessels for dense -mesh effect in the early stage. For long segment dissections, tandem partial overlapped stenting was deployed at 47 vessels.
DSA (51/91,56%), CTA (38/91, 41.8%), and HR-MRI (2/91, 2.2%) has been followed up with a mean time of 17.2±15.4 months (5–77).
Clinically, no mortality and morbidity were perioperatively occurred (Table 2). The TIA was occurred in three patients during the operation (3/74, 4.1%). Asymptomatic embolism was found in two patients (2/74, 2.7%). All patient mRS score no increased at discharge. Pre-, postoperative and follow-up mRS was collected and compared in Fig. 2. The clinical outcomes after stenting among different vessel locations (two CCA, 86 extracranial ICA, and six VA) had no statistical difference (p>0.05).
Angiographically, success rate of stent deployment is 90/91 (99%). Only one patient with very tortuous dissecting segment, partial remodeling was reconstructed via large-profile stent due to difficult accessibility. Complete reconstruction of dissections in 86 vessels (86/91, 94.5%, Fig. 3) and minor remnant dissecting segment in five (5/91, 5.5%) were revealed at follow up. Severe stenosis of the intra-stent was re-stented in one patient (1/91, 1.1%, Fig. 4).
Unplanned 30-day hospital readmissions of symptomatic carotid and VA dissection reach the 9.08% and 8.43% respectively while primary cause of readmission was ischemic stroke [11]. Though the previous study not distinguish location of dissection (intra vs. extracranial), outcomes still contribute understanding of the development of carotid and vertebral dissections. Several studies presume that endovascular stenting is reserved for cases failed medical therapy [11]. However, in this present study, stent remodeling of extracranial supra-aortic arterial dissection with high-grade stenosis (>70%) or occlusion, and aneurysmal dilations was proved safely and efficaciously with 99% technical success rate. Pham et al. [12] reported 99% technical success rate and 1.3% procedural complication rate in 140 patients (153 carotid dissecting vessels) consistent with our results. Stent remodeling technique for mostly dissections was simply reached. However, for multiple lumen dissection of the ICA, super-selection of the real lumen using dual microcatheter or microwire was key for the repair of the intimal tear. Overlapping stents is useful in patients with long dissected segments. In addition, for the tortuous cervical segment dissection of the ICA, stent accessibility is priority. Dual wire or Gateway balloon assisted navigation of the guiding catheter could afford more robust support force. Guiding catheter-assisted stent deployment in this present study is important.
The potential risks associated with endovascular stenting was questioned. Periprocedural complication rate of 1.3% was reported in 140 patients treated endovascularly for ESADs all of which were asymptomatic complication [12]. Prior studies revealed low procedural risks and high technical success rate [1,8,17], however, long-term stability was unclear. In this study, no morbidity and mortality rate proved the safety of this stent remodeling technique for dissection at post-operation and follow-up. Two occlusion cases compensated by the external carotid artery and posterior communicating artery also presented with a contralateral dissection. Complete occluded dissection recanalization requires higher technical skill and poses greater risk of complications. However, similar occlusive segment recanalization is relative safer than atherosclerotic lesions considering that the occlusive segments are located in the cervical segment of the ICA presenting limited range and relatively straight route [20]. More importantly, real lumen identification is the key step to reconstructing a dissecting segment. In our study, dual microwire and microcatheter application proved useful in confirming the real lumen. Many studies do not use distal protection device filters [4,6,16]. Considering the risk of worsening the dissection with these devices, meanwhile no associated atherosclerotic plaque presented in this type patients. However, two patients were suffered from asymptomatic embolic migration due to thrombus in the dissecting segment in our data. Therefore, in some multiple lumen or large thrombus volume dissection segment, distal protection device filters should be prepared to avoid distal embolism. Stents were normally deployed without balloon pre-angioplasty except for pure very severe stenotic or occlusive lesions. Stent remodeling technique is a feasible approach for supra-aortic dissections.
Cervical artery dissections could induce several severe clinical consequences with 7.4% mortality rate [18]. Farouk et al. [5] investigated endovascular treatment is useful for acute ischemic stroke induced by carotid dissection. Delgado et al. [3] reported that endovascular treatment of selected cases of patients presents good short-term clinical results for acute and non-acute phases of carotid dissections. All patients with non-acute phase of cervical arterial dissection in this present study demonstrate improvement resolution of presenting symptoms. No patient displayed any new neurological deficits at clinical follow-up in this present study.
Stent related complications mainly include intra-stent restenosis and stent fracture were reported from other diseases of the atherosclerotic stenosis [10]. The use of antiplatelet could decreased the incidence of intra-stent restenosis. No stent fracture and relatively low intra-stent restenosis rate (1/91, 1.1%) were determined by nature of the lesion in this data. Stent ends should be deployed at straightened segment of the carotid or VA to avoid angle increasement. If stent tip was released in artery curve, which damage the intima of the artery via the stent end tip and might subsequently lead to restenosis. One severe restenosis was occurred in overlapped stent site of previous occlusive lesion induced by nature of the lesion. Side-effects of the radiotherapy include endothelial damage and the decline of endothelial repair capacity [19]. In patients presenting with dissections remodeling, 89.1% were successfully well reconstructed, with the remaining 5.5% showing minor remnant dissection particular in intracranial stents cases. Proper stent type deployed right location contribute the remodeling effect. Stent type choice has a significant learning curve. For CAD lesions lower than ICA petrosal segment, a carotid stent was recommended. For higher than ICA cervical segment and VAD lesions, intracranial stents could be selected. Rahal et al. [13] reported five CAD patients using the Enterprise stent treatment in tortuous ICA. However, kinking and malposition of stent were revealed due to the low radial force of the intracranial stents. Koge et al. [7] investigated two cases of highly tortuous carotid artery dissection after large-profile carotid stent straightening with a peripheral guidewire. Similar to this present study, the large-profile carotid stent was more high radial force than intracranial stents to straighten the tortuous ICA. ESADs with severe stenosis, occlusion and/or pseudoaneurysm present potential risk of stroke, even in the chronic stage. Meanwhile, stenting has proven long-term reliability, with relatively low procedural risk.
This study has certain limitations. First, this retrospective observational clinical study has 12 years large time span which means that methods of diagnosis, materials, and equipment have changed and these are factors that might affect outcomes. Second, small sample size was visualized. Acute phase ESAD cases were not included in this study due to high basal NIHSS score, more unilateral lesion and relatively poor prognosis. Further larger studies with corresponding improvement will be contribute to elucidate the efficacy and safety of stent remodeling technique.
Notes
ACKNOWLEDGMENTS
This study was supported by the National Nature Science Foundation of China (Grant No. 81771242).
References
1. Ansari SA, Thompson BG, Gemmete JJ, Gandhi D. Endovascular treatment of distal cervical and intracranial dissections with the neuroform stent. Neurosurgery. 62:636–646. discussion 636-646. 2008.

2. Biller J, Sacco RL, Albuquerque FC, Demaerschalk BM, Fayad P, Long PH, et al. Cervical arterial dissections and association with cervical manipulative therapy: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 45:3155–3174. 2014.

3. Delgado F, Bravo I, Jiménez E, Murías E, Saiz A, Vega P, et al. Endovascular treatment in the acute and non-acute phases of carotid dissection: a therapeutic approach. J Neurointerv Surg. 9:11–16. 2017.

4. Edgell RC, Abou-Chebl A, Yadav JS. Endovascular management of spontaneous carotid artery dissection. J Vasc Surg. 42:854–860. discussion 860. 2005.

5. Farouk M, Sato K, Matsumoto Y, Tominaga T. Endovascular treatment of internal carotid artery dissection presenting with acute ischemic stroke. J Stroke Cerebrovasc Dis. 29:104592. 2020.

6. Fava M, Meneses L, Loyola S, Tevah J, Bertoni H, Huete I, et al. Carotid artery dissection: endovascular treatment. Report of 12 patients. Catheter Cardiovasc Interv. 71:694–700. 2008.

7. Koge J, Iwata T, Mizuta S, Nakamura Y, Matsumoto SI, Yamada T. Successful carotid artery stenting of a dissected, highly tortuous internal carotid artery after straightening with a peripheral microguidewire. J Clin Neurosci. 53:265–268. 2018.

8. Lee YJ, Ahn JY, Han IB, Chung YS, Hong CK, Joo JY. Therapeutic endovascular treatments for traumatic vertebral artery injuries. J Trauma. 62:886–891. 2007.

9. Leys D, Bandu L, Hénon H, Lucas C, Mounier-Vehier F, Rondepierre P, et al. Clinical outcome in 287 consecutive young adults (15 to 45 years) with ischemic stroke. Neurology. 59:26–33. 2002.

10. Li MKA, Tsang ACO, Tsang FCP, Ho WS, Lee R, Leung GKK, et al. Long-term risk of in-stent restenosis and stent fracture for extracranial vertebral artery stenting. Clin Neuroradiol. 29:701–706. 2019.

11. Mehta T, Patel S, Male S, Parikh R, Mehta K, Lakshminarayan K, et al. Unplanned 30-day hospital readmissions of symptomatic carotid and vertebral artery dissection. J Stroke. 20:407–410. 2018.

12. Pham MH, Rahme RJ, Arnaout O, Hurley MC, Bernstein RA, Batjer HH, et al. Endovascular stenting of extracranial carotid and vertebral artery dissections: a systematic review of the literature. Neurosurgery. 68:856–866. discussion 866. 2011.

13. Rahal JP, Gao B, Safain MG, Malek AM. Stent recanalization of carotid tonsillar loop dissection using the enterprise vascular reconstruction device. J Clin Neurosci. 21:1141–1147. 2014.

15. Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 344:898–906. 2001.

16. Schulte S, Donas KP, Pitoulias GA, Horsch S. Endovascular treatment of iatrogenic and traumatic carotid artery dissection. Cardiovasc Intervent Radiol. 31:870–874. 2008.

17. Seifert T, Klein E, Legat-Wallner S, Krenn U, Brussee H, Lueger A, et al. Bilateral vertebral artery dissection and infratentorial stroke complicated by stress-induced cardiomyopathy. J Neurol Neurosurg Psychiatry. 79:480–481. 2008.

18. Urasyanandana K, Songsang D, Aurboonyawat T, Chankaew E, Withayasuk P, Churojana A. Treatment outcomes in cerebral artery dissection and literature review. Interv Neuroradiol. 24:254–262. 2018.

Fig. 1.
Schematic representation of stent remodeling technique for extracranial supra-aortic dissections : dissections in cervical segment of internal carotid artery (A). 0.35 wire cross the dissecting segment (B). A 6 F envoy guiding catheter cross the dissecting segment (C). Precise stent (6×40 mm) was in position and retrieving the guiding catheter partially (D). Precise stent was deployed (E). Angiography showed stenosis at the proximal of the stent (F). Navigated the guiding catheter into the previous stent lumen (G and H). Acculink 6–8×40 mm stent was deployed tandemly and overlapped partially with previous stent (I). Postoperative and 12 months working projection view revealed favorable remodeling of the dissecting segment respectively (J and K).
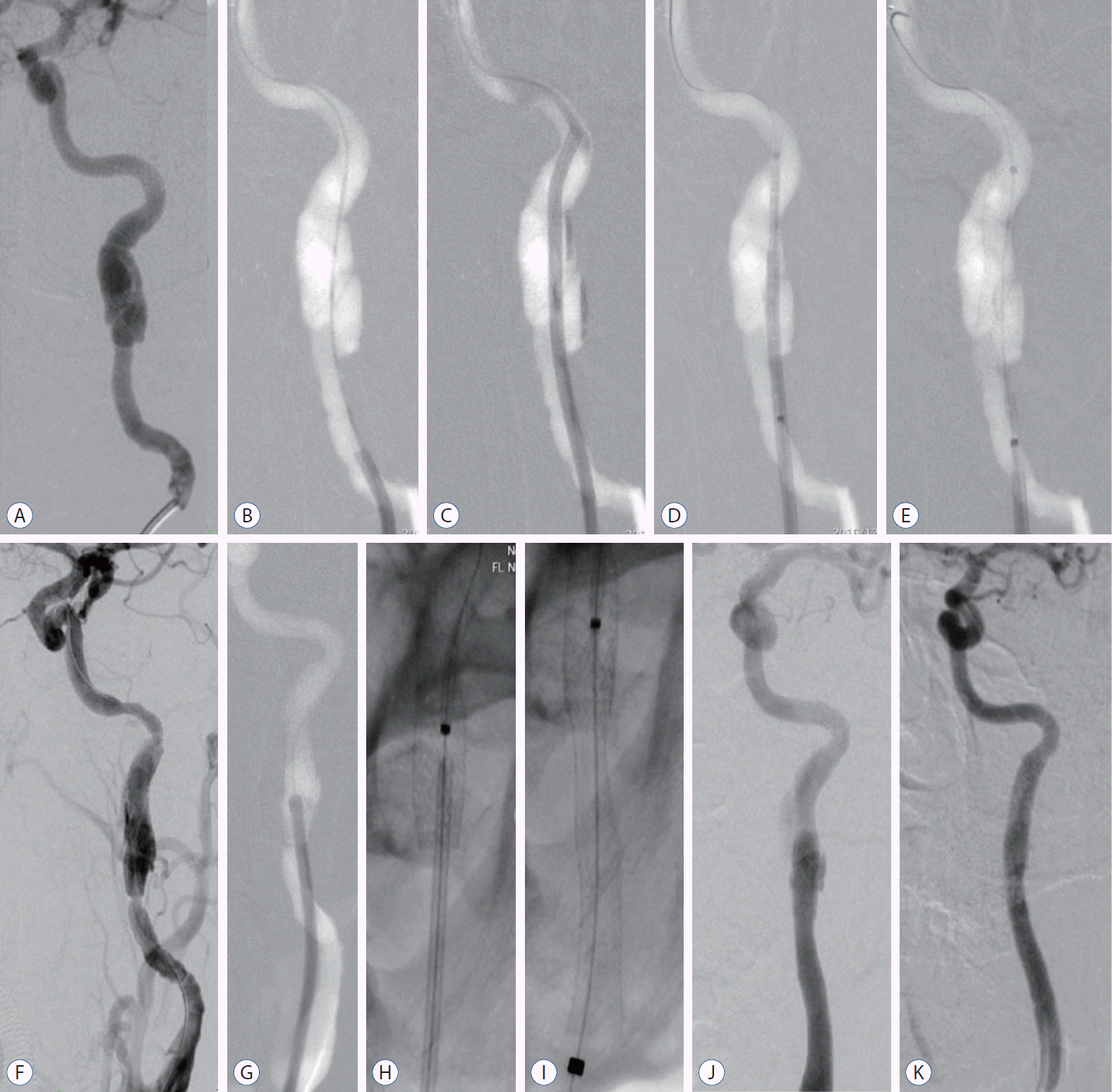
Fig. 2.
Clinical outcomes comparison among preoperative, postoperative and follow up modified Rankin Scale (mRS).
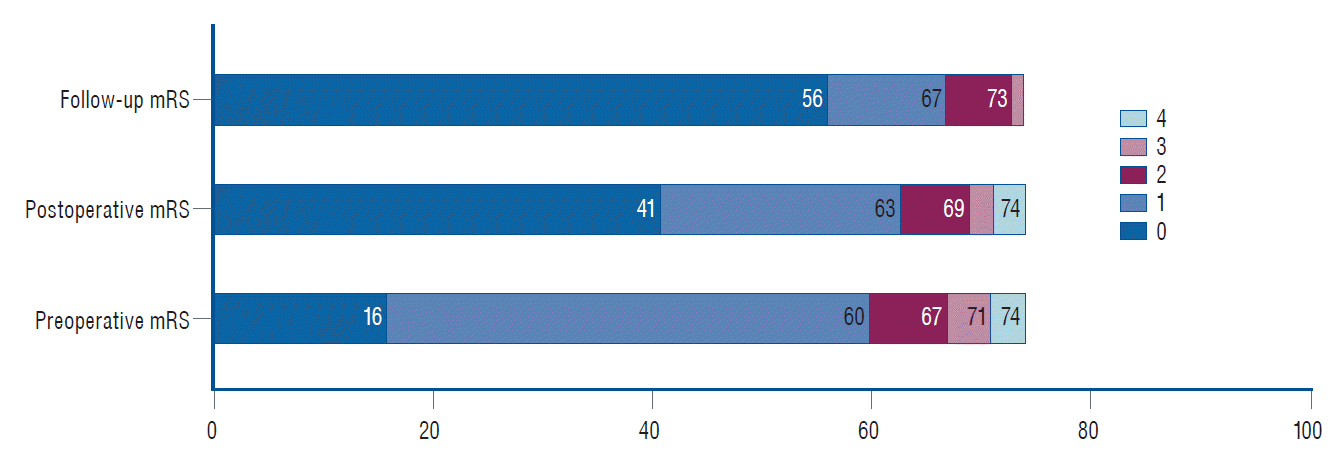
Fig. 3.
Forty-seven years old male patient with sudden weakness of right limbs and aphasia 3 months ago. Digital subtracted angiography revealed that internal carotid artery (ICA) cervical dissecting aneurysm and stenosis of dissections (A). Postoperative angiography showed segment of the ICA dissections remodeled by two Wingspan stents (4.5×20 mm and 4×20 mm; B). The ICA dissections were repaired well at 12 months follow-up (C).
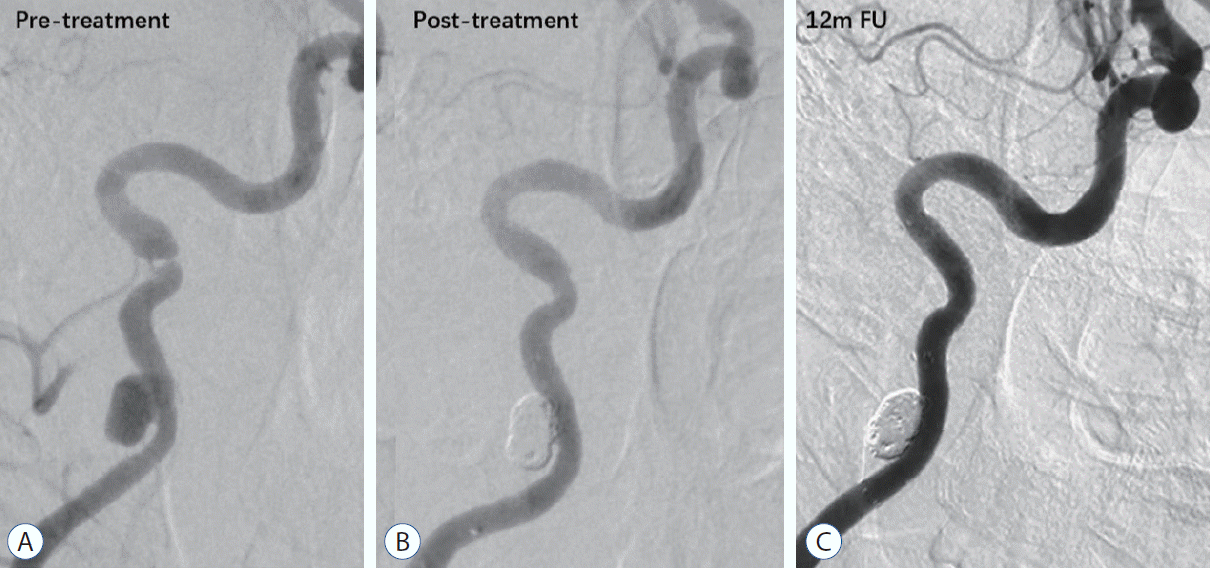
Fig. 4.
Forty-three years old male patient with dizziness, diplopia and drowsiness 10 days ago. Diffusion-weighted imaging demonstrated bilateral acute infarctions in basal ganglion region (A). DSA revealed that occlusive cervical segment of the left ICA compensated by external carotid artery (B). Postoperative angiography showed segment of the ICA dissections remodeled by Wingspan 3.5×15 mm, Precise 6×40 mm and Acculink 6–8×40 mm stent (C). Three months follow-up CTA revealed patency of the dissecting segment (D). Forty-five months follow-up CTA and DSA demonstrated the asymptomatic intrastent severe restenosis (white arrow; E and F). Postoperative angiography showed the intrastent restenosis remodeled by Precise 6×30 mm stent (G). DSA : digital subtracted angiography, ICA : internal carotid artery, CTA : computational tomography angiography.
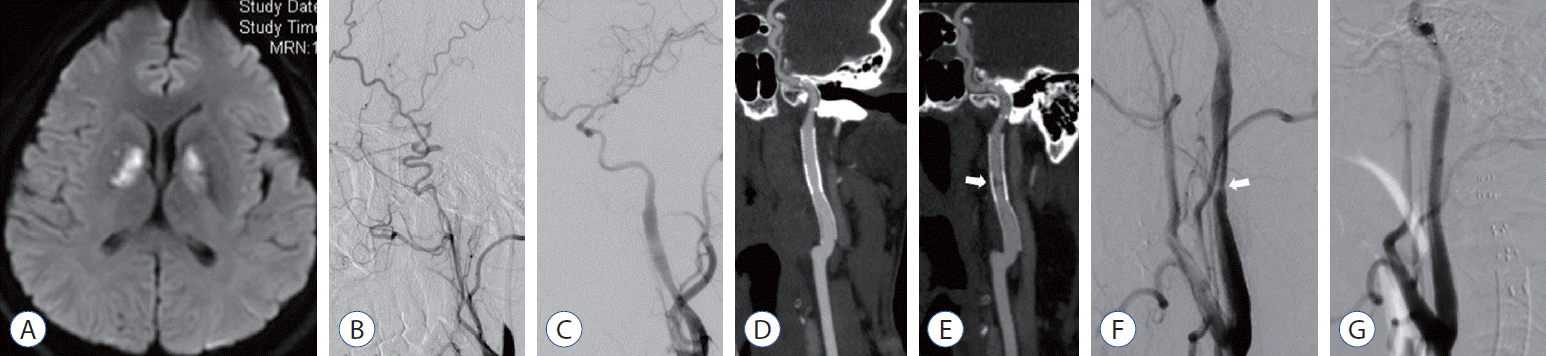
Table 1.
General characteristics of the 74 patients with 91 extracranial arterial dissections




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download