Abstract
Objective
We conducted this study with the aim of predicting the biological behavior of meningiomas, and determining the benefits of associating histological subtype and grade with the expression of proliferative markers and tumor suppressor proteins.
Methods
The study included 29 patients with primary intracranial and intraspinal meningioma diagnosed in the pathology laboratory of Konya City Hospital between January 2014 and December 2020. Clinicopathological characteristics of the patients including parameters such as age and gender were obtained from the hospital records. Histopathological findings were obtained by re-evaluating the preparations stained with Hematoxylin-Eosin, which were extracted from the archive, and by evaluating new sections obtained from paraffin blocks of patients stained with Ki67, p53, and p57 immunohistochemical stains.
Results
A moderate correlation was found between tumor size and Ki67 proliferation index (PI) (p=0.003, r=0.530). There was no significant difference between grade I and grade II tumors in terms of p53 (p=0.184) and p57 (p=0.487) expressions. There were higher levels of Ki67 PI in grade II tumors. The histological subtypes of the tumor had no significant difference with Ki67 PI (p=0.018), p53 (p=0.662), and p57 (p=0.368) expressions.
Conclusion
In order to obtain more definitive results, there is a need for studies, which are conducted with a greater number of patients and in multiple centers, and in which a long prospective follow-up is planned. The combination of histological, surgical, and imaging markers could make a more sensitive tool for predicting recurrence, and this could also be tested in future studies.
Meningiomas are common tumors mainly with slow growth patterns. On the other hand, they may rarely exhibit invasive growth, and they are long-lasting diseases [17]. Meningiomas are primary central nervous system (CNS) tumors related to the dura mater arising from the meningothelial cells of the arachnoid [3,4,14]. They account for approximately 36.8% of intracranial primary tumors. They affect the female more than the male, and they have an incidence with a greater increase after the age of 65 [4]. In 2016, World Health Organization (WHO) reported that variants of meningiomas are classified in two groups as meningiomas with aggressive behavior and low risk of recurrence. Besides, it classified the meningiomas as grade I, II, and III [4,8]. Atypical meningiomas (AM), which are characterized by increased mitotic activity and a higher risk of recurrence, are classified as WHO grade II; and anaplastic meningiomas, which are aggressive and exhibit malignant behavior, are classified as WHO grade III. Grading has important implications for patient management, and it has been demonstrated to be associated with the prognosis. Contrast-enhanced computed tomography (CT) and gadolinium-enhanced magnetic resonance imaging (MRI) are the preferred methods for the initial diagnosis since meningiomas are extra-axial tumors with a dense tissue structure. During surgery, the neurosurgeon aims to remove the entire tumor detected through MRI [10]. While the treatment of grade I tumors is performed by surgery, grade II and III tumors are treated with surgery, radiotherapy, and chemotherapy [14]. Even though recurrences are observed after the surgery, the surgical method is a priority in treatment [11,17].
When evaluating the biological behavior of meningiomas, mitotic and cell proliferation indices, the intensity of angiogenesis, the effect of sex steroid hormones, inflammatory markers, tumor suppressors and, genetic and immunological alterations should be considered in addition to histopathological findings [4]. Genetic alterations in atypical and anaplastic meningiomas and specific histological subtypes have not been fully defined yet. Nonetheless, various molecular studies have examined alterations in many tumor suppressor genes and oncogenes [12]. There is an increasing clinical need for identifying predictors of more and better recurrence and tumor progression compared to the current scope of histological grading and resection. Since immunohistochemistry has been a routine practice in pathological diagnosis for decades, investigation of predictive immunohistochemical markers is important for facilitating the procedure [2,3].
p57 is a member of the CIP/KIP family of cyclin-dependent kinase (CDK) inhibitors along with p21 and p27. It plays a role in the management of cell cycle, cell differentiation, apoptosis, tumorigenesis, angiogenesis, migration, and invasion. CDK inhibitors are necessary for the normal progression of the cell cycle [7,9]. The only phase where the decision on the cycle depends on external stimuli is the G1 phase within the four phases of the cell-division cycle (G1/S/G2/M). While progression to the S phase and proliferation occurs only in the presence of growth factors, the cells enter a smooth G0 phase in their absence. G1 phase is divided into early and late G1 phases with the “G1/S checkpoint”. The early and late phases of G1 are managed by enzymatic complexes formed by CDKs and the associated cyclins. If the cells pass this point, they are determined to progress through an entire cycle, and they no longer need growth factors to complete the division process. The expression of p57 is rather limited in adult tissues. p57 is considered a tumor suppressor gene due to its ability to inhibit proliferation. The importance of p57 in suppressing cancer is emphasized by its mutation/inactivation in Beckwith-Wiedemann Syndrome, which is a cancer susceptibility syndrome [6]. In some human malignancies such as lung cancer, hepatocellular carcinoma, and bladder cancer, the expression of p57 decreases significantly, thereby confirming its involvement during tumorigenesis [9,13].
Ki67 is a nuclear protein associated with a mitotic activity. It is involved in all phases of the cell cycle, except for the G0 phase. This protein has been proven to be effective in determining proliferative activity in the cell in meningiomas. It is routinely used in clinical practice due to its association with mitotic index and histopathological grade in meningiomas [4,11,14]. Ki67 proliferation index (PI) has a strong correlation with tumor growth, recurrence, and disease-free survival in various tumors, including meningiomas [3].
p53, a nuclear phosphoprotein, is an important tumor suppressor that acts mainly on the cell cycle in the maintenance and repair of DNA by affecting the apoptosis of mutated cells. Therefore, the loss of mutation-induced p53 function leads to the accumulation of DNA mutations, dysregulation of the cell cycle, and apoptotic induction. A high proportion of cells with the mutated p53 protein indicates greater tumor aggression [4,11]. Loss of p53 suppressive function is a common status in the development of a wide variety of human tumors, increasing genetic instability and metastatic potential [12].
We conducted this study to predict the biological behavior of meningiomas and determining the benefits of associating histological subtype and grade with the expression of proliferative markers and tumor suppressor proteins.
The study was approved by KTO Karatay University, Ethics Committee of Research with Non-Pharmaceutical Products and Non-Medical Devices (IRB number : 2021/043).
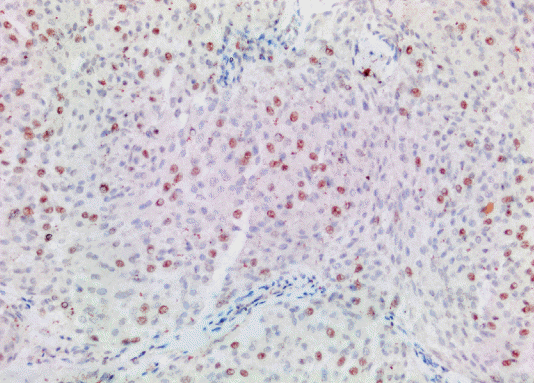
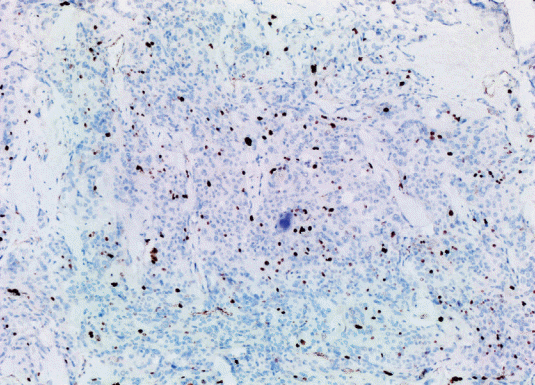
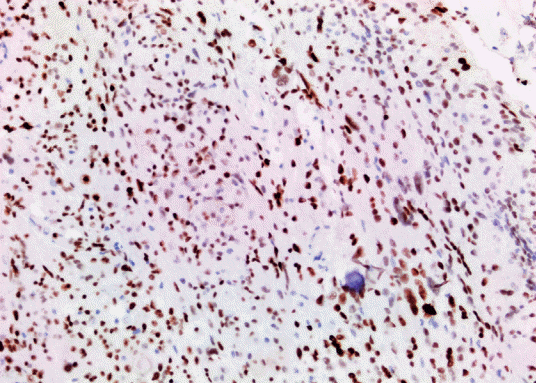
The study included 27 patients with primary intracranial and two patients with primary intraspinal meningioma diagnosed in the pathology laboratory of our center between January 2014 and December 2020. Clinicopathological characteristics of the patients including parameters such as age and gender were obtained from the hospital records. The diameter measured on MRI was considered as the tumor size. Histopathological findings were obtained by re-evaluating the preparations extracted from the archive (Table 1). Histological subtype and grade were determined according to 2016 WHO classification (Fig. 1). Rabbit monoclonal antibody p57 (1 : 150 diluted, Cell Marque Tissue Diagnostic; SIGMA-ALDRICH, Roclin, CA, USA), rat monoclonal antibody p53 (1 : 100; Thermo Scientific, Runcorn, UK), rabbit monoclonal antibody Ki67 (1 : 200; Thermo Scientific) were used for immunohistochemical evaluation. Primary antibodies were visualized using an avidin-biotin-peroxidase complex method. Diaminobenzidine was used as the substrate. Sections were counterstained by Mayer’s hematoxylin. Brown nuclear immunoreactivity was considered as positive staining for Ki67, p57, and p53. Based on various cut-off data in the literature, p57 expression was classified as ≤5% positive cells and >5% positive cells (Fig. 2) [7]. Ki67 PI was recorded as the percentage of positively stained tumor nuclei per 1000 tumor cells. Cell counts were performed under ×400 magnification in areas with maximum immunoreactivity (hot spot) (Fig. 3) [12]. p53 was evaluated with a scoring system where no expression was scored as 0, mild expression was scored as 1 (<10%), medium expression was scored as 2 (10–50%), strong expression was scored as 3 (>50%) (Fig. 4) [2]. The section of colon adenocarcinoma was used as positive control for p53. Tonsil sections were used as a positive control for Ki67 and placental tissue was used as a positive control for p57. All patients were evaluated according to the histological subtype of meningioma, localization, and degree of malignancy, gender, and the density of the expressions of Ki67, p53, and p57 proteins.
The statistical analyses were performed using SPSS ver. 15.0 for Windows (SPSS, Chicago, IL, USA). The Shapiro-Wilk test was used for examining the continuous variables with normal and abnormal distributions, while the one-way analysis of variance was used for the normally distributed continuous variables. The Kruskal-Wallis test was used for the abnormally distributed continuous variables. When the Kruskal-Wallis test indicated statistically significant differences, the causes of those differences were determined using a Bonferroni-adjusted Mann-Whitney U test. The continuous variables were presented as the mean±standard deviation. The Spearman correlation analysis was used to study the correlations between measurements. Survival was assessed using Kaplan-Meier analysis. Statistical significance was considered at p<0.05.
The mean age was 57.76±16.01. There were 22 female (75.9%) and seven male (24.1%) patients. Mean Ki67 PI was 7.14±6.26 in female patients, and 5.43±5.62 in male patients. Mean p53 expression was 1.73±1.07 in female patients, and 2.14±0.69 in male patients. The mean p57 expression was 3.41 ±5.27 in female patients, and 3.43±3.69 in male patients. There was no significant relationship between gender and the expression of Ki67 PI (p=0.566), p53 (p=0.409) and p57 (p=0.533) (Table 2). A moderate correlation was found between tumor size and Ki67 PI (p=0.003, r=0.530). There was no correlation between the expressions of p53 (p=0.666) and p57 (p=0.664), and the tumor size. Among the patients, 21 (72.4%) were grade I, eight (27.6%) were grade II. There were no grade III tumors among our series. The mean Ki67 PI was 4.43±4.28 in grade I tumors, and 12.75±6.11 in grade II tumors. The mean p53 expression was 2.00±0.89 in grade I tumors and 1.38±1.18 in grade II tumors. The mean p57 expression was 3.81±5.29 in grade I tumors and 2.38±3.66 in grade II tumors. There was no significant difference between grade I and grade II tumors in terms of p53 (p=0.184) and p57 (p=0.487) expressions. There were higher levels of Ki67 PI in grade II tumors (Table 3).
Among the tumors, 11 were meningothelial meningiomas (MM), eight were AM, five were transitional meningiomas (TM), three were psammomatous meningiomas (PM), and two were fibrous meningiomas (FM). The mean Ki67 PI was 12.75±6.11 in AMs, 3.00±1.41 in FMs, 4.82±5.17 in MMs, 4.67 ±6.35 in PMs, and 4.00±1.58 in TMs. The mean p53 expression was 1.38±1.18 in AMs, 1.91±0.94 in MMs, 2.33±1.15 in PMs, and 2.00±1.00 in TMs. There was no p53 expression in FM. The mean P57 expression was 2.38±2.38 in AMs, 1.00± 1.41 in FMs, 3.18±5.96 in MMs, 6.33±6.80 in PMs, and 4.80± 4.08 in TMs. The histological subtypes of the tumor had no significant difference with Ki67 PI (p=0.018), the expressions of p53 (p=0.662), and p57 (p=0.368) (Table 4). Tumor localization was classified as infratentorial (IT) and supratentorial (ST). Two tumors located in the thoracic region were classified in the IT group. STs were located in the frontal (n=16), temporal (n=4) and parietal (n=2) lobes, respectively. Of these, 11 were on the right side, nine were on the left side, and two were in the midline. Two of the ITs were located in the right cerebellum, two in the left cerebellum, one in the pons, and two in the spinal cord at the thoracic level. The mean Ki67 PI in STs was 4.14±3.80, and it was 7.55±6.47 in ITs. The mean p53 expression in STs was 2±0.81, and it was 1.77±1.06 in ITs. Mean p57 expression was 1.71±1.70 in STs, and it was 3.95±5.44 in ITs. The localization of meningiomas had no significant difference with Ki67 PI (p=0.122), and the expressions of p53 (p=0.709) and p57 (p=0.672).
The median follow-up time of the patients was 1.82 years for progression free survival (PFS) and 1.87 years for overall survival (OS). The correlation between the Ki67, p53, and p57 expression of the tumors and PFS (log-rank, p=0.411, p=0.433, and p=0.057, respectively) or OS was not significant (log-rank, p=0.710, p=0.258, and p=0.058, respectively).
Meningiomas are primary CNS tumors related to the dura mater arising from the meningothelial cells of the arachnoid [4,14]. They account for approximately 36.8% of intracranial primary tumors, affecting the female more than the male. Tumor recurrence is an important and infrequent case; however, we have limited knowledge about the predisposing factors. There is a risk of recurrence by 7–25% in WHO grade I, by 30–50% in WHO grade II, and by 50–95% in WHO grade III meningiomas [3,8]. Since meningiomas generate space-occupying lesions, they present with clinical symptoms such as headache, epilepsy, and blurred vision. Surgery is currently the main treatment for meningioma. Radiotherapy, chemotherapy and biotherapy are included in other treatment approaches. Factors affecting the formation and development of meningiomas have not been clarified yet [17].
p57 is mainly expressed in skeletal muscles, cardiac muscle, brain, lung, kidney, pancreas, testicles, and placental tissue. On the other hand, it is expressed during fetal organogenesis, particularly in differentiating cells. Also, transcriptional and translational downregulation of p57 is common in many human cancers, including lymphoid malignancies. There are studies strongly suggesting that this protein has similar tumor-suppressing effects in various cancers [9,15]. It acts in the regulation of cellular processes such as apoptosis, differentiation, and tumorigenesis [7]. Various studies have demonstrated that low expression of p57 is associated with poor prognosis in lung, oral, esophageal, gastric, colorectal, hepatocellular, and pancreatic malignancies. Downregulation of p57 has been proven to accelerate cellular proliferation, growth, and invasion in hepatocellular carcinomas. Similarly, decreased p57 expression has been reported to be associated with survival in the short term in recent studies on breast cancer. Downregulation of p57 in human cancers and its importance in targeted therapy is a current subject of investigation [7,9]. Zhang [17] observed that the expression of CD133, p57, and HSF1 detected by real time-polymerase chain reaction in meningioma tissue was significantly higher compared to the normal brain tissue. They suggested that these markers could lead to a new basis for the treatment of meningioma due to their role in the growth and development of the meningioma. Originally identified as a CDK inhibitor, p57 has been proven to have different cellular functions, as well as cell cycle inhibition such as the regulation of cell migration and cell differentiation. Today, p57 is found to play a key role in coordinating the stress-induced cellular response, driving the cell to both apoptosis and cellular senescence. These findings raise the question of would the re-evaluation of p57 be a promising approach to cancer treatment [13]? In addition, clinical studies suggest that the absence of p57 eliminates the pro-apoptotic function of anticancer therapy, leading to poor prognosis due to the development of drug resistance [9].
However, in our study, we did not find a significant relationship between the expression of p57 and, tumor size, gender, histological subtype, tumor localization, and tumor grade in meningiomas.
Various immune markers have been proposed to predict recurrence. The cell proliferation marker Ki67, which is one of these markers, appears to be a good marker for recurrence/regrowth in grade I meningiomas, and only Ki67 is a part of the routine clinical practice. The selection of the block for immunostaining may have some effect on Ki67 PI due to the heterogeneity of the tumor. This may result in the underestimation of the actual proliferative potential of the tumor. This overlap between tumor grades may reduce the benefits of Ki67 PI in the grading of meningioma in a limited number of cases, especially when a relatively low Ki67 PI is obtained. Therefore, Ki67 PI should be correlated with clinical and radiological findings before evaluation [2,3].
Ki67 PI is a useful complement to histomorphology in the diagnosis of meningiomas. While the values of ≥4% are associated with slow behavior, Ki67 PI >4% indicates a more advanced tumor grade [2]. In the study by Abry et al. [1], the cutoff value for Ki67 PI was 3% of the stained cells. After reaching this cutoff value, there were significant differences in Ki67 when analyzed according to histopathological subtypes of meningiomas. A higher proportion of Ki67 positive meningiomas were transitional (32.1%), while fibrous (11.7%) and meningothelial (80.0%) meningiomas were more frequently Ki67-negative [1,3,11]. We used the same cut-off value in our study; however, we did not find a significant difference between histological subtypes and Ki67 PI. A cut-off value has not been defined for Ki67 in many other tumor types, including meningiomas. When defining a cut-off value, different results may be obtained, as there is no consensus for a high and low proliferative index in tumor cells. Therefore, future studies should focus on the definition of the optimal cut-off value for evaluating the immunohistochemical expression of cell cycle markers [7]. In a meta-analysis evaluating Ki67 positivity in grade I meningiomas, the authors obtained a mean value of about 3% for Ki67 PI in recurrent tumors. In their study, Ki67 PI was examined immunohistochemically in 580 patients; and it was observed that Ki67 PI was increased in recurrent meningiomas. Pavelin et al. [11] reported significant positive correlations in both Ki67 and p53 with tumor size. They concluded that they potentially affected the development and growth of meningiomas [11]. In our series, we found a moderate correlation between tumor size and Ki67 PI. Similar to the literature, we found that Ki67 PI was higher in grade II tumors. On the other hand, we did not find a significant relationship between Ki67 PI in meningiomas and, gender, histological subtype, and tumor localization.
p53 is the most commonly studied tumor suppressor gene and protein; however, the mechanisms of its tumor suppressor effects have not been fully understood yet. Most of the studies addressing the role of p53 in meningiomas clearly demonstrate that there is a significant relationship between p53 positivity and the grade of meningioma. Several authors have reported that positive p53 protein expression is mainly associated with malignant histology. They also noted that p53 protein loss was more common in elderly patients, while there was a higher expression of this tumor suppressor in younger patients. Nonetheless, statistical analysis indicated that p53 expression levels were not significantly correlated with certain age groups. The protective function of the p53 protein decreases with age and may lead to the failure of cells in responding to stress as well as an increased incidence of tumors in elderly populations [12]. Kumar et al. [5] observed 0%, 19%, and 23.1% p53 immunopositivity in grade I, grade II, and grade III meningiomas, respectively. We also did not observe p53 expression in two patients with fibrous meningioma (WHO grade I). In the study conducted by Cho et al. [2], the p53 immunoreactivity demonstrated in grade I, grade II, and grade III meningiomas were 9.5%, 72.7%, and 88.9%. This indicated that the immunoreactivity of p53 significantly increased according to the advance in histological grade. While p53 expression was observed in 71.4% of recurrent meningiomas, this rate was only 10.5% in non-recurrent meningiomas. p53 gene mutation was observed in 62.5% of grade II meningiomas and 25% of grade III meningiomas. On the other hand, there was no mutation in any of the grade I meningiomas. In conclusion, Cho et al. [2] pointed out that p53 immunopositivity and TP53 gene mutation were associated with the prognosis of meningiomas, and they act as markers indicating the progression of meningiomas. Also, Yang et al. [16] reported a positive correlation between the overexpression of p53 and, malignant progression, tumor recurrence, and histological grades of meningioma. However, in our study, we did not find a significant relationship between the expression of p53 and, tumor size, gender, histological subtype, tumor localization, and tumor grade in meningiomas. p53 antibody, which is used in routine practice, does not differentiate between wild and mutant types of protein. In recurrent cases, p53 is more likely to be mutant and ineffective, thereby contributing to tumor growth and recurrence [3]. Our results are different from the results in the literature. This may be explained by the fact that p53 immunoreactivity does not differentiate according to wild and mutant types of protein.
The correlation between the p57, Ki67 and p53 expression of the meningiomas and PFS or OS was not significant. These results indicate that p57, Ki67 and p53 expression cannot be recommended as a prognostic parameter for meningioma.
With the onset of the COVID-19 pandemic, our hospital started to work as a pandemic hospital. Therefore, the number of patients is low. At the same time, there is no grade III meningioma in our patient group and, it was not possible to conduct a study with more prognostic markers due to high costs.
To obtain more definitive results, there is a need for studies, which are conducted with a greater number of patients and in multiple centers, and in which a long prospective follow-up is planned. The combination of histological, surgical, and imaging markers could make a more sensitive tool for predicting recurrence, and this could also be tested in future studies.
Notes
Informed consent
Informed consent was obtained from all individual participants included in this study.
References
1. Abry E, Thomassen IØ, Salvesen ØO, Torp SH. The significance of Ki-67/MIB-1 labeling index in human meningiomas: a literature study. Pathol Res Pract. 206:810–815. 2010.

2. Cho H, Ha SY, Park SH, Park K, Chae YS. Role of p53 gene mutation in tumor aggressiveness of intracranial meningiomas. J Korean Med Sci. 14:199–205. 1999.

3. Csonka T, Murnyák B, Szepesi R, Bencze J, Bognár L, Klekner A, et al. Assessment of candidate immunohistochemical prognostic markers of meningioma recurrence. Folia Neuropathol. 54:114–126. 2016.

4. De Carvalho GTC, Da Silva-Martins WC, De Magalhães KCSF, Nunes CB, Soares AN, Tafuri LSA, et al. Recurrence/regrowth in grade I meningioma: how to predict? Front Oncol. 10:1144. 2020.
5. Kumar S, Kakkar A, Suri V, Kumar A, Bhagat U, Sharma MC, et al. Evaluation of 1p and 14q status, MIB-1 labeling index and progesterone receptor immunoexpression in meningiomas: adjuncts to histopathological grading and predictors of aggressive behavior. Neurol India. 62:376–382. 2014.

6. Lam WW, Hatada I, Ohishi S, Mukai T, Joyce JA, Cole TR, et al. Analysis of germline CDKN1C (p57KIP2) mutations in familial and sporadic beckwith-wiedemann syndrome (BWS) provides a novel genotypephenotype correlation. J Med Genet. 36:518–523. 1999.

7. Latic D, Pejic S, Savic S, Loncar Z, M Nikolic I, Nikolic G, et al. Cyclin D1 and p57 expression in relation to clinicopathological characteristics and overall survival in patients with renal cell carcinoma. J BUON. 24:301–309. 2019.
8. Louis DN, Perry A, Reifenberger G, Von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 131:803–820. 2016.

9. Madhuri TK, Tailor A, Haagsma B, Coley H, Butler-Manuel S. Relevance of immunohistochemical expression of p57kip2 in epithelial ovarian carcinoma- a systematic literature review. J Ovarian Res. 5:46. 2012.

10. Mehidine H, Refregiers M, Jamme F, Varlet P, Juchaux M, Devaux B, et al. Molecular changes tracking through multiscale fluorescence microscopy differentiate meningioma grades and non-tumoral brain tissues. Sci Rep. 11:3816. 2021.

11. Pavelin S, Becic K, Forempoher G, Mrklic I, Pogorelic Z, Titlic M, et al. Expression of Ki-67 and p53 in meningiomas. Neoplasma. 60:480–485. 2013.

12. Pećina-Šlaus N, Kafka A, Vladušić T, Tomas D, Logara M, Skoko J, et al. Loss of p53 expression is accompanied by upregulation of beta-catenin in meningiomas: a concomitant reciprocal expression. Int J Exp Pathol. 97:159–169. 2016.

13. Rossi MN, Antonangeli F. Cellular response upon stress: p57 contribution to the final outcome. Mediators Inflamm. 2015:259325. 2015.

14. Telugu RB, Chowhan AK, Rukmangadha N, Patnayak R, Phaneendra BV, Prasad BC, et al. Histopathological and immunohistochemical evaluation of meningiomas with reference to proliferative markers p53 and Ki-67. J Clin Diagn Res. 10:EC15–EC19. 2016.

15. Tesio M, Trumpp A. Breaking the cell cycle of HSCs by p57 and friends. Cell Stem Cell. 9:187–192. 2011.

16. Yang SY, Park CK, Park SH, Kim DG, Chung YS, Jung HW. Atypical and anaplastic meningiomas: prognostic implications of clinicopathological features. J Neurol Neurosurg Psychiatry. 79:574–580. 2008.

17. Zhang X. Expression, correlation and prognostic significance of CD133, P57 and HSF1 in meningioma. Eur Rev Med Pharmacol Sci. 21:4600–4605. 2017.
Fig. 1.
Histopathological subtypes of meningioma, staining with Hematoxylin-Eosin. A : Meningothelial type (×100). B : Transitional type (×100). C : Psammomatous type (×200). D : Atypical type (×400).
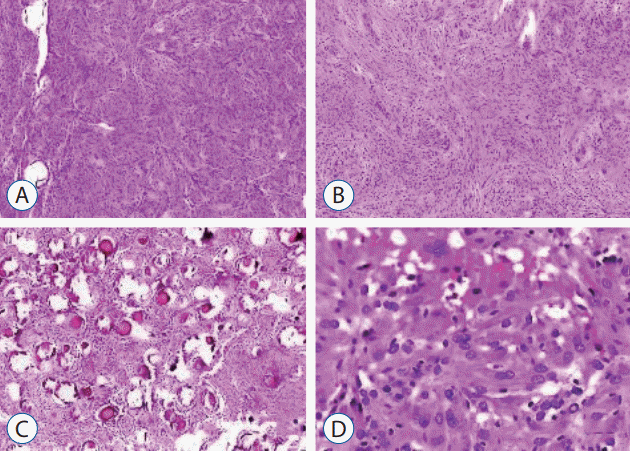
Table 1.
Clinicopathological characteristics of 29 patients with meningioma
Table 2.
Relationship between gender and immunohistochemical parameters
| Male (n=7) | Female (n=22) | p-value* | |
|---|---|---|---|
| Ki67 PI | 5.43±5.62 | 7.14±6.26 | 0.566 |
| p53 | 2.14±0.69 | 1.73±1.07 | 0.409 |
| p57 | 3.43±3.69 | 3.41±5.27 | 0.533 |
Table 3.
The relationship between tumor grade and immunohistochemical parameters
| Ki67 PI | p53 | p57 | |
|---|---|---|---|
| Grade | |||
| I | 4.43±4.28 | 2.00±0.89 | 3.81±5.29 |
| II | 12.75±6.11 | 1.38±1.18 | 2.38±3.66 |
| p-value* | <0.001 | 0.184 | 0.487 |
Table 4.
The relationship between histopathological subtypes and immunohistochemical parameters
| Histopathologic subtype | Ki67 PI | p53 | p57 |
|---|---|---|---|
| Atypical (n=8) | 12.75±6.11 | 1.38±1.18 | 2.38±2.38 |
| Meningothelial (n=11) | 4.82±5.17 | 1.91±0.94 | 3.18±5.96 |
| Psammmomatous (n=3) | 4.67±6.35 | 2.33±1.15 | 6.33±6.80 |
| Transitional (n=5) | 4.00±1.58 | 2.00±1.00 | 4.80±4.08 |
| Fibrous (n=2) | 3.00±1.41 | - | 1.00±1.41 |
| p-value* | 0.018 | 0.662 | 0.368 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download