Abstract
Objective
Dermoid cysts are uncommon in spinal cord tumors, and the phenomenon of their spontaneous rupture into the syrinx cavity is quite rare. We aimed to analyze the imaging characteristics and etiologies, and propose some surgical strategies, for this uncommon phenomenon.
Methods
We retrospectively reviewed 14 cases with spinal dermoid cysts that ruptured into the cervical and thoracic syrinx cavity. There were six male and eight female cases, aged 21 to 46 years, who had lipid droplets in the syrinx cavity from C1 to L3. The dermoid cysts were always located at the conus. Based on patients’ complaints, clinical manifestations, and imaging results, we adopted tumor excision and/or syrinx cavity aspiration in one stage or multiple stages.
Results
Three patients had only a syrinx cavity aspiration surgery due to a history of dermoid cyst excision. Eight patients had dermoid cyst resection and syrinx cavity aspiration in one stage. One patient was operated upon in two stages due to the development of new symptoms at nine months follow-up. Two patients underwent only tumor resection since they did not show similar symptoms or signs caused by the cervicothoracic syrinx. The axial magnetic resonance imaging indicated that the lipid droplets were always not at the center but were eccentric. The clinical effect was satisfactory during the follow-up period in this group.
Spinal dermoid cysts, which are composed of keratinized squamous epithelium, are covered with connective tissue with islands of dermis containing sebaceous glands and hair follicles. They arise from ectopic embryonic remnants of ectoderm and mesoderm in the spinal canal during neural tube closure of the fetal life [4,16]. However, they can also be acquired through iatrogenic procedures like spinal surgery, lumbar puncture, or trauma, since the dermal components could be introduced into the subarachnoid space.
It is reported that spinal dermoid cysts account for less than 1% of all spinal cord tumors [6]. They are rare, slow-growing, and benign lesions, that can be located intramedullary, intradural-extramedullary, and extradurally [12]. The lumbosacral spinal canal is the segment that is most commonly affected with a spinal dermoid cyst. The conus medullaris and cauda equina are always involved. The least common affected segment is the cervical and upper thoracic regions. They can also be associated with split cord malformation, tethered cord, myelomeningocele, bony malformation, dermal sinus tract, or hypertrichosis.
The dermoid cyst rupture into the syrinx cavity or central canal alone is a rare occurrence and has been reported in few studies [2,3]. Interestingly, no fatty droplets were found in the subarachnoid space in the cases presented. We propose that the lipid droplets are mostly located in the syrinx cavity, not entirely confined to the central canal, which is associated with the development of the dermoid cyst. In this study, we reported on 14 cases with ruptured spinal dermoid cysts into the cervicothoracic intramedullary syrinx. Compared to previous studies, and yet most of them are case reports, we involved relatively larger cases and tried to analyze the etiology of ruptured spinal dermoid cysts, the imaging characteristics and clinical results, and proposed some surgical strategies.
The study was approved by the Institutional Research Ethics Committee of Chinese PLA General Hospital (No. [2016]1024). We retrospectively studied 14 patients with ruptured spinal dermoid cysts between May 2012 and September 2020. All patients, four males, and eight females, aged 21 to 46 years, had lipid droplets in the syrinx cavity from C1 to L3, and the dermoid cysts were located at the conus. The neurological examination also revealed no cranial nerve dysfunction. The most common clinical presentation was paresis of the extremities; other symptoms or signs such as gait problems, spastic weakness, excretion difficulties, back pain, radiculopathy, and myelopathy were also noted. On T1-weighted sequence magnetic resonance imaging (MRI), the fat manifested as high signals. However, it could be suppressed into low signals on a fat-suppression MRI, which is an excellent tool to evaluate the characteristic information (Figs. 1-3; cases 1, 3, and 6). Neurological functions were evaluated using the modified McCormick grading (mMG) system, from grade I to grade IV (from the best neurologic function to the worst state). The follow-up time ranged from 3 to 93 months and was characterized by routine neurological examination, regular MRI, and computerized tomography (CT) scan.
According to the chief complaints and physical signs, combined with the character of imageology results, we adopted different surgical strategies. Tumor resection and syrinx cavity aspiration were performed in one stage or stages. The patients were placed in the prone position if the syrinx aspiration was conducted in the region of the cervical or cervicothoracic junction. The Mayfield 3-point skull stabilization and fixation device was used. A dorsal midline incision was made until a pale, grayish, or yellowish dermoid cyst was exposed. Evacuation of the contents, management of the capsule wall, and near-total resection was carried out under neurophysiological monitoring. We tried to strip the majority of the capsule to prevent tumor recurrence. In the intramedullary cervicothoracic syrinx cavity filled with lipid droplets, we performed about 10–20-mm length midline myelotomy. Ivory or yellow lipid droplets flowed out (Fig. 4) : aspiration of the liquid fatty material, repeated irrigation, and aspiration was conducted. For the patients with tethered cord simultaneously, we released the spinal cord and nerve roots and/or resected the thick filum terminale. Intraoperative monitoring was used to prevent possible neurological injuries during the surgery.
Fourteen patients were enrolled in this study, and the demographic and clinical characteristics are shown in Table 1. Eight patients (cases 1, 4, 6, 7, 11, 12, 13, and 14) underwent both dermoid cyst resection and syrinx aspiration in one stage based on their symptoms and signs associated with the upper and lower extremities, including the back, bladder, and/or bowel dysfunction. Three patients (cases 3, 5, and 9) had a reported history of thoracolumbar dermoid cyst excision at different clinical centers. Before surgery, they had both the dermoid cyst and cervicothoracic syrinx cavity but only dealt with the dermoid cyst at that time. They were admitted to our department and had syrinx aspiration when the symptoms of neck pain, back pain, upper extremities numbness, or motor deficits occurred. One patient (case 2) was operated upon in two stages since he had back pain and right upper extremity paralysis at nine months follow-up. Cases 8 and 10 underwent only tumor resection; they had no symptoms caused by the cervicothoracic syrinx cavity and no tumor recurrence when MRI was assessed at several follow-ups. Nine patients had tethered cord released and resected the thick filum terminale. Neurological function was evaluated by mMG; in this group, the worst was case 7, who improved from grade III to grade II at three months follow-up. All others had an improvement compared with their preoperative state. No patient presented with symptoms associated with chemical meningitis after the resection. The longest follow-up time was 93 months. Three patients refused reoperation when MRI indicated conus tumor recurrence (21.4% recurrence rate) because they did not experience apparent symptoms or worse signs.
The spinal dermoid cysts are typically unilocular and contain yellowish-brown or yellow viscous fluid, with creamy contents of different types of fat, such as lipid metabolites, crystals of cholesterol, or keratin. They are formed as a result of defecation at the time of neural tube closure between the third and fifth gestational weeks [15].
Until now, the mechanism of dermoid cyst rupture into the syrinx cavity remains unclear, and further research is needed. The central spinal canal is generally regarded as a rudimentary structure that is obliterated after birth in about 70–80% of the general population, which means that it is not normally open and only accepted as a potential space in the adults [7,14]. The lipids are not only confined to the central canal, which is a potential non-patent space, and a syringomyelia cavity may have probably pre-existed with the dermoid cyst; furthermore, the association of intramedullary tumors with syringomyelia is well-recognized [8,13]. The syrinx cavity may not communicate with the central canal in most cases. On an MRI scan, the cavity was always eccentric on axial images (Fig. 5). That is similar to syringomyelia in Chiari malformation. The intramedullary fluid was thought to be located in the enlarged central canal in the past; however, some experts proposed that the syrinx cavity was not the enlarged central canal in the majority of patients in recent years [11]. The syringomyelia cavity caused by the developmental dermoid cyst might explain the entry of dermoid contents into the syrinx. Since the contents have low specific gravity, they always float on top of the cerebral spinal fluid. The collapse of the tumor may result in intramedullary entrapment of these fat droplets, leading to the development of a cavity [14], which cannot be absorbed automatically. This feature is different from the fluid in Chiari syringomyelia, where the cavity could shrink or even disappear after the surgery. Based on this phenomenon, after the excision of the lumbosacral dermoid cyst, a follow-up aspiration of the cervicothoracic intramedullary lipid droplets is recommended when there are associated symptoms or signs.
MRI is the essential radiologic modality in diagnosing spinal dermoid cyst and observing the syrinx cavity. Generally, dermoid cysts are hyperintense on T1 MRI, and on fat-suppressed sequences, the signal is suppressed, and this could help to confirm the diagnosis. However, not all dermoid cysts displayed a high T1-weighted signal, and they sometimes demonstrate heterogeneous signals based on the complicated tumor contents such as hair, bone, cartilage, or other debris. The ruptured dermoid cysts into the cervicothoracic syrinx cavity could be seen as multiple, discrete, rounded, or oval, hyperintense signals on T1-weighted images. They are suggestive of tiny intramedullary lipomas. We found that most syrinx cavities on axial images were eccentric; this could support that the dermoid cyst ruptured into the syrinx cavity. Dissemination of high signal T1-weighted lipid droplets in the syrinx following the rupture is characteristic of ruptured dermoid cysts [2]. CT is a useful tool to observe whether there is bone or calcification in the tumor or spinal dysraphism, and the Hounsfield unit can be used to differentiate lipid and pneumocephalus when small lipids disseminated into the cranial cavity is observed.
In the differential diagnosis, many pathologies such as astrocytoma, ependymoma, lipoma, epidermoid cyst, or arachnoid cyst, can be partially differentiated using MRI. Every pathology has its characteristics, the difference between the dermoid and epidermoid cyst, which is difficult to be differentiated on images. These cysts come from the absence or presence of cutaneous type adnexal structures and are two major variants of ectodermal-derived neuraxis cysts. Both are produced by the inward displacement of ectoderm during embryonic development. The possibility to find dermoid cysts in the lumbosacral region is more common, owing to the closure of the ectoderm to the neural tube starts from the cranial end [5]. The spinal location of dermoid cysts occurs more often than epidermoid cysts, which are more common in the cranium.
Most patients with spinal dermoid cysts were admitted to the hospital due to the mass effect on neural tissues, which caused low back pain or radiculopathy. When the cyst ruptures into the cervicothoracic region, the patients may present with neck pain, back pain, or upper extremity motor deficit. They occur predominantly in the lumbosacral region (60%), involving the conus medullaris and cauda equina, and are quite rare in the cervical (5%) and the thoracic regions (10%) [16]. Unlike the rupture of the cranial dermoid cyst, which always manifests as acute presentation, rupture and subsequent release of lipid contents into the syrinx cavity are usually asymptomatic or only present with nonspecific and mild symptoms. The rupture of an intracranial dermoid cyst is frequently symptomatic. The existence of lipid droplets in the ventricular and subarachnoid spaces may cause ventriculitis, arachnoiditis, vasospasm, aseptic chemical meningitis, or hydrocephalus. Clinical symptoms of acute rupture may include vertigo, headache, nausea, vomiting, hemiplegia, mental changes, or even coma [1,8].
Due to a limited number of papers that have reported on ruptured dermoid cyst into the syrinx cavity, there are no unified standards for surgery. In this study, eight patients were treated in one stage, where both excision of the dermoid cyst and aspiration of the intramedullary lipid droplets were performed. The decision was based on the patient’s chief complaints, clinical manifestations, and imaging results. If the complaints were bladder or bowel dysfunction, sensory or motor deficits of the lower extremities, which were caused by the lumbosacral tumor, we recommend that resection surgery be considered first. When the patients’ complaints were paresthesia or paralysis of the upper limbs, neck, and chest region, which were caused by the cervicothoracic intramedullary lipid syrinx, we suggest that syrinx aspiration should be performed. If the patient did not have these associated lumbosacral complaints, but only presented with the cervicothoracic symptoms or signs, we still suggest that the tumor should be resected and combined with the aspiration surgery. That is different from the reverse situation; the intramedullary lipid syrinx could be managed conservatively when there were no associated complaints, or they are quite tiny without a space-occupying effect. The reason is that the origin of intramedullary lipid droplets is from the ruptured dermoid cyst, so the tumor needs to be excised even without associated symptoms. Of course, if the cervicothoracic and lumbosacral complaints exist simultaneously, the tumor excision and lipid droplets aspiration should be performed in one stage, not in stages.
In deciding on the level of the cervicothoracic syrinx region to operate on, we chose the largest diameter segment on the MRI. After myelotomy, yellow or white lipid flowed out. Afterward, aspiration and repeated irrigation were performed. The septa always existed in the cavity, and it was challenging to aspirate the entire intramedullary lipid completely when the syrinx cavity was extended over a long area. Close follow-up and stage-by-stage surgery might be considered in this condition [11]. The dermoid cyst was excised by midline myelotomy. Then evacuation of the contents, including pearly colored, white-yellowish pultaceous material mixed with hair, or even bone and calcified materials, were removed with special care. Then was followed by the excision of the capsule wall of the cyst, which was tightly adherent to the spinal cord but could not be separated completely. Leakage of tumor contents into the subdural region should be inhibited during the surgery to prevent possible postoperative aseptic chemical meningitis. Comparing to intramedullary ependymoma, the contents of the dermoid cyst are more complex, while the low-grade ependymoma could always reach to en bloc complete resection. With respect to intramedullary astrocytoma, it has ill-defined tumor margins with no or mild enhancement, partial rection is necessary to preserve neurological status with higher rates of recurrence. Neurophysiological monitoring was conducted to protect neurological functions. Attempt to complete resection may lead to permanent neurological damage [9]. However, we still need to make an effort to resect the capsule wall under intraoperative monitoring to reduce the risk of recurrence [10].
The limitation of this study is that this is a retrospective study, and due to its rare incidence of this disease, the majority of the earlier papers were individual case reports, whereas this study included a relatively larger number of patients. Furthermore, the exact etiology of ruptured spinal dermoid cysts is not clear and requires further research.
Ruptured spinal dermoid cyst into the syrinx cavity is a rare entity. The lipid droplets were always not at the center but eccentric on axial images. The lipid droplets were filled in the syringomyelia cavity, not only confined to the central canal, which may be caused by the developmental tumor. Based on the patients’ chief complaints and associated neurological signs, we adopted different surgical strategies and underwent tumor resection and/or syrinx aspiration at one stage or in stages.
Notes
Author contributions
Conceptualization : AS, JW; Data curation : CC, RL, HG, BT, HW, MS, GG; Formal analysis : CC, BT, RL; Funding acquisition : AS, HG; Methodology : AS, JW, CC, BT; Project administration : AS, JW, CC; Visualization : AS, JW, CC, BT; Writing - original draft : CC, BT; Writing - review & editing : CC, BT, JW, AS
ACKNOWLEDGMENTS
We are grateful to the research grant from the Beijing Municipal Science & Technology Commission, PR China, No. Z171100001017140 (to AJS) and the Science Foundation of Military Medical Research and Clinical Research Foundation of PLA General Hospital in China, No. 2017FC-TSYS-2026.
References
1. Altay H, Kitiş O, Calli C, Yünten N. A spinal dermoid tumor that ruptured into the subarachnoidal space and syrinx cavity. Diagn Interv Radiol. 12:171–173. 2006.
2. Bishnoi I, Bishnoi S, Gahlawat N, Bhardwaj L, Duggal G, Singal G, et al. Management of a rare case of intraventricular ruptured dermoid cyst and chemical meningitis. Br J Neurosurg. 19:1–4. 2018.

3. Calabrò F, Capellini C, Jinkins JR. Rupture of spinal dermoid tumors with spread of fatty droplets in the cerebrospinal fluid pathways. Neuroradiology. 42:572–579. 2000.

4. Falavigna A, Righesso O, Teles AR. Concomitant dermoid cysts of conus medullaris and cauda equina. Arq Neuropsiquiatr. 67(2A):293–296. 2009.

5. Fernández-de Thomas RJ, Vicenty-Padilla JC, Sánchez-Jiménez JG, Labat EJ, Carballo-Cuello CM, De Jesús O, et al. Obstructive hydrocephalus and chemical meningitis secondary to a ruptured spinal epidermoid cyst. World Neurosurg. 132:173–176. 2019.

6. Goyal A, Singh D, Singh AK, Gupta V, Sinha S. Spontaneous rupture of spinal dermoid cyst with disseminated lipid droplets in central canal and ventricles. J Neurosurg Sci. 48:63–65. 2004.
7. Karaaslan B, Ülkü G, Ucar M, Demirdağ TB, İnan A, Börcek AÖ. Intramedullary dermoid cyst infection mimicking holocord tumor: should radical resection be mandatory?-a case report. Childs Nerv Syst. 32:2249–2253. 2016.

8. Karadag D, Karagülle AT, Erden A, Erden I. MR imaging of a ruptured intraspinal dermoid tumour with fat droplets in the central spinal canal. Australas Radiol. 46:444–446. 2002.

9. Kasliwal MK, Sinha S, Sharma BS, Garg A. Symptomatic central canal rupture heralding the presence of an asymptomatic conus dermoid. J Neurooncol. 84:39–40. 2007.

11. Liu T, Deng X, Xu Y, Xin Y. Spinal dermoid cyst with spontaneous rupture into the syrinx cavity alone. World Neurosurg. 118:e395–e404. 2018.

12. Petit-Lacour MC, Lasjaunias P, Iffenecker C, Benoudiba F, Hadj Rabia M, Hurth M, et al. Visibility of the central canal on MRI. Neuroradiology. 42:756–761. 2000.

13. Samii M, Klekamp J. Surgical results of 100 intramedullary tumors in relation to accompanying syringomyelia. Neurosurgery. 35:865–873. discussion 873. 1994.

14. Sanaullah M, Mumtaz S, Memon AA, Hashim AS, Bashir S. Intramedullary dermoid cyst with relatively atypical symptoms: a case report and review of the literature. J Med Case Rep. 7:104. 2013.

Fig. 1.
Case 6. Preoperative magnetic resonance imaging (MRI) revealed a tethered conus lesion with heterogeneous hyperintense on lumbosacral T1-weighted images, and homogeneously spindle or linear signal on the cervical and thoracic spinal cord (A-C). Postoperative MRI showed subtotal resection of the conus dermoid cyst, and lipid droplets reduced significantly in the cervical and thoracic regions (D-F).
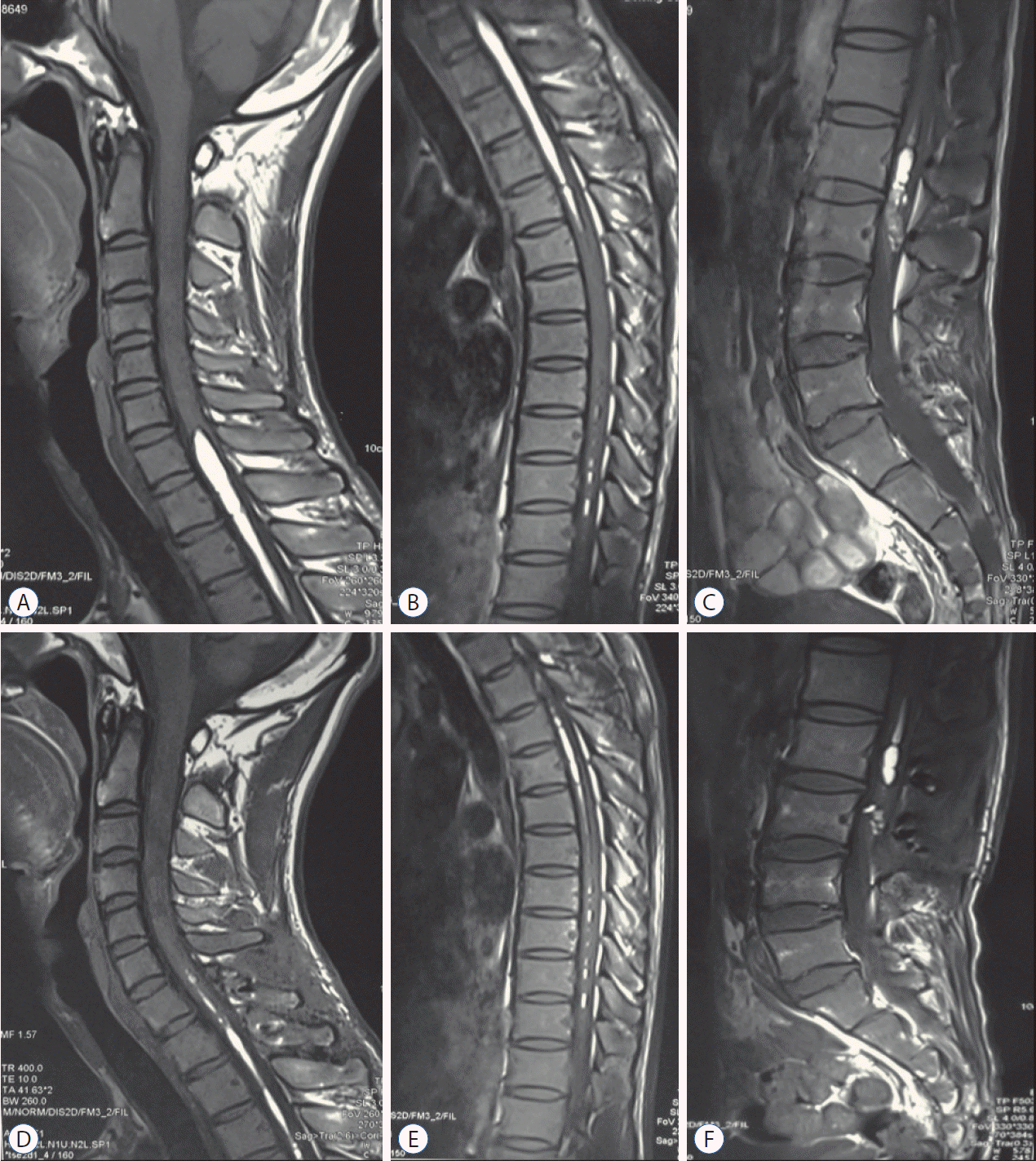
Fig. 2.
Case 3. The preoperative whole spine magnetic resonance imaging images revealed C7-T6 intramedullary lipid droplets (short T1-weighted and long T2-weighted signal on A and B), which were suppressed on fat-suppressed sequences (C). The heterogeneous signals were seen at the level of conus medullaris, indicating a possible dermoid cyst. After conus tumor surgery at another clinical center, the patient was admitted to our hospital and had a T1 syrinx cavity aspiration surgery. The cervical intramedullary lipid droplets almost disappeared, and the conus dermoid cyst showed recurrent signs even though the patient did not have apparent symptoms at 72 months follow-up (D and E).
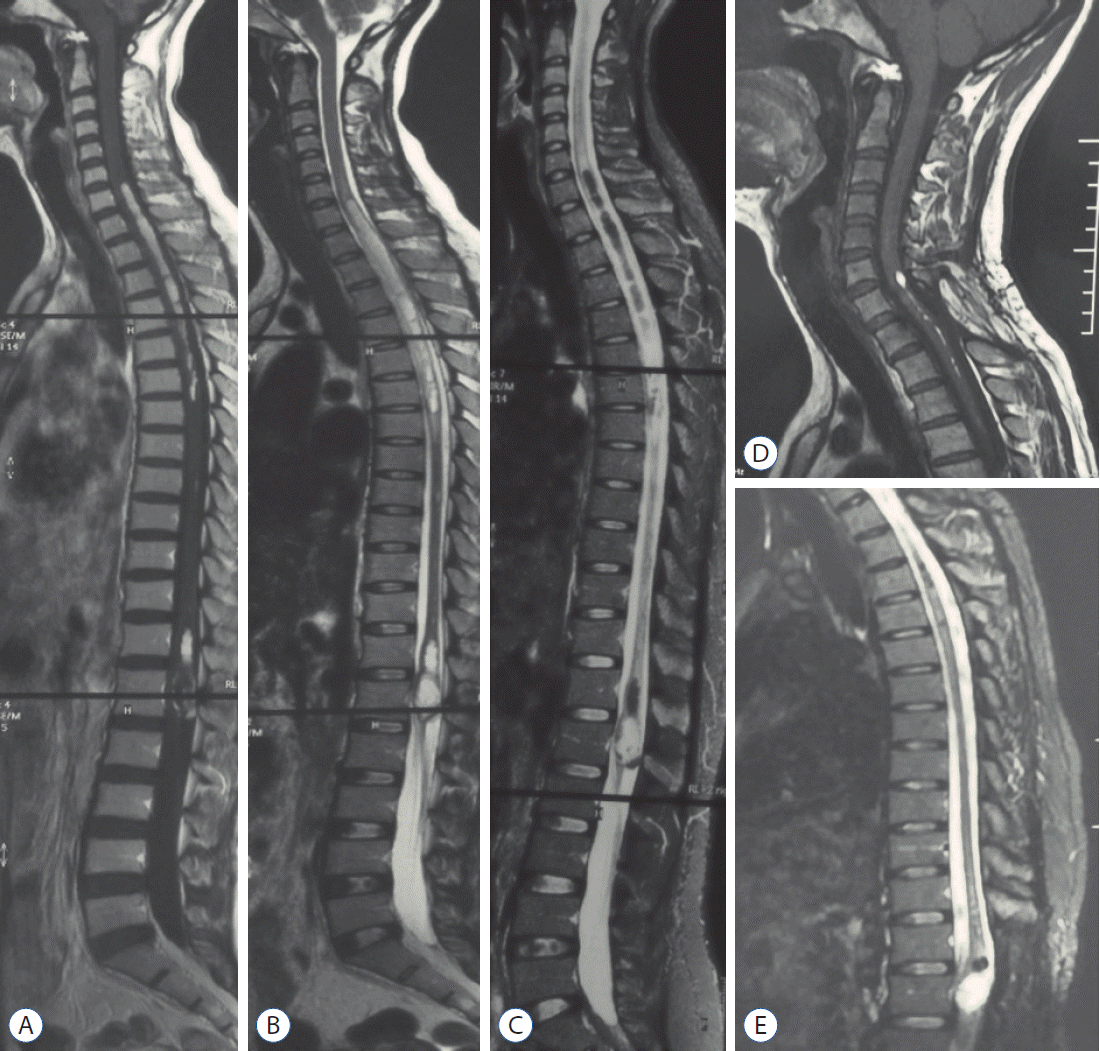
Fig. 3.
Case 1. Preoperative magnetic resonance imaging (MRI) showed short-T1 cervical intramedullary lesion and mixed-signal lumbar dermoid cyst (A and B). Postoperative MRI revealed the tumor subtotal resection in one stage (C and D).
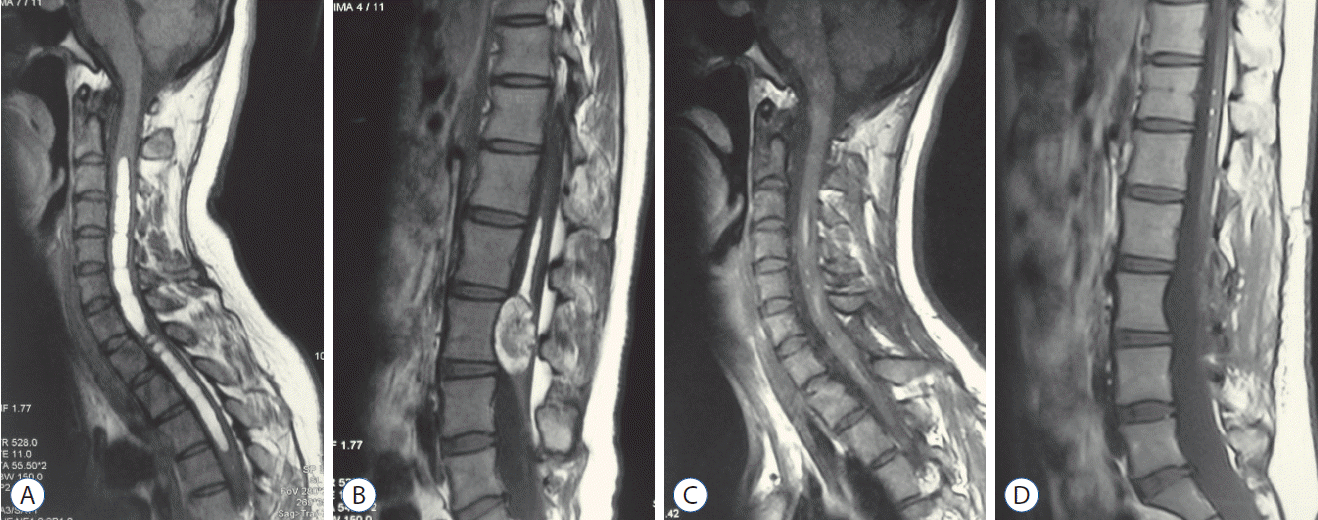
Fig. 4.
Intraoperative views showed ivory lipid droplets flowed out (A; red arrow) after cervical spinal cord myelotomy; revealed yellowish dermoid cyst contents exposure (B; red arrow).
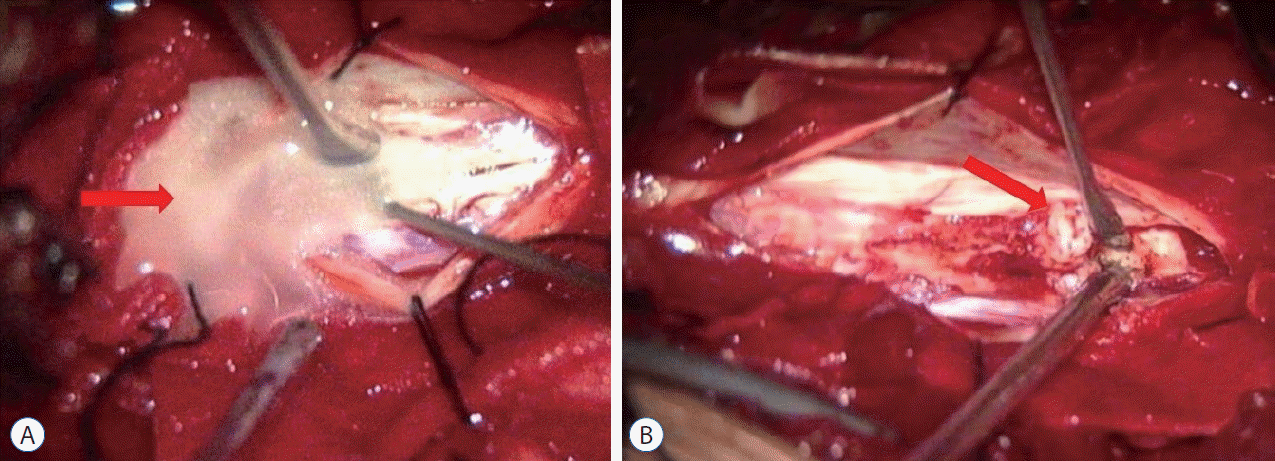
Fig. 5.
A-F : The axial images indicated that the lipid droplets were always eccentric, not only confined to the central canal, and can be distributed in any direction.
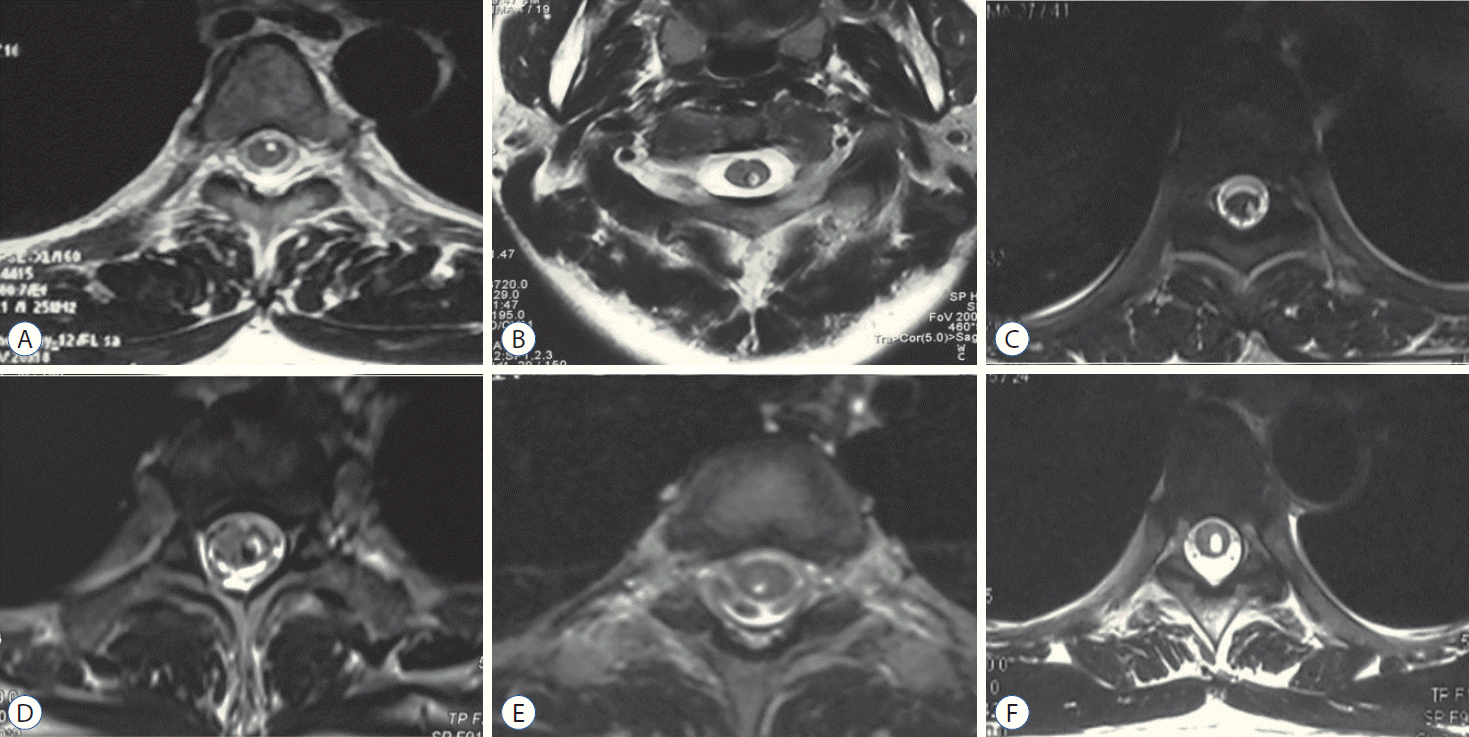
Table 1.
Demographic and clinical profile of the patients




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download