Abstract
Development of de novo dural arteriovenous fistula (DAVF) at a different site after resolution of an initial DAVF, is rare. Here we report two cases, which we encountered in our hospital. A 68-year-old woman presented with pulsatile tinnitus on the left side. Cerebral angiography demonstrated a left anterior condylar confluence (ACC) DVAF and she underwent transvenous embolization. Four years after this treatment, she presented with tinnitus on the left side, and cerebral angiography revealed a right DAVF around the sinus of the lesser sphenoid wing. Another 69-year-old woman presented with left-sided orbital bruits, chemosis, and conjunctival hyperemia. Cerebral angiography showed left cavernous sinus (CS) DAVF, for which she underwent transvenous embolization for CS DAVF. One year later, she developed a left ACC and transverse-sigmoid sinus (TSS) DAVF.
Dural arteriovenous fistula (DAVF) is an abnormal arteriovenous shunt disease, which occurs within the dura mater, and comprises approximately 10% of intracranial vascular malformations [9]. DAVFs are usually treated by the disconnection of venous drainage via transarterial or transvenous embolization [10]. It is a pathophysiological rarity when a second DAVF develops in another location following endovascular embolization of the initial DAVF. We report two cases of de novo DAVFs after transvenous coil embolization for the initially located DAVF.
This study was approved by the appropriate Institutional Review Board of Hamamatsu University School of Medicine (IRB No. CRB4180008) and adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from the patients.
A 68-year-old woman presented to our hospital with pulsatile tinnitus on the left side. Cerebral angiography showed left anterior condylar confluence (ACC) DAVF, and the fistula was treated with transvenous coil embolization via the left internal jugular vein; and the tinnitus subsequently disappeared (Fig. 1A and B). Follow-up magnetic resonance imaging (MRI) showed ACC DAVF obliteration and she completed 3 years of follow-up after the treatment, with no complaints.
Four years after the treatment, the patient presented again with tinnitus on the left side, and diagnostic angiography demonstrated that a DAVF on the right side around the sinus of the lesser sphenoid wing (SLSW), which was fed mainly by the accessory meningeal artery (Fig. 1C). Retrograde leptomeningeal venous drainage was refluxed from the SLSW to the superficial middle cerebral vein via a bridging vein (Fig. 1D). The DAVF drained from the SLSW through the sphenopetrosal vein (SPV) and superior petrosal sinus (SPS) into the inferior petrosal sinus (IPS) via the cavernous sinus (CS). Internal carotid artery angiography showed no connection between the SLWS and CS and absence of venous congestion in the venous phase.
A microcatheter was inserted through the transvenous approach into the varix of the SLWS via the SPV and SPS via the CS. The varix and the shunt point were tightly packed with coils, and final angiography confirmed complete remission of the DAVF (Fig. 1E). Her symptoms resolved after coil embolization, and MRI showed SLSW DAVF obliteration.
A 69-year-old woman presented with left-sided orbital bruits, chemosis, and conjunctival hyperemia. Cerebral angiography showed left CS DAVF, and the fistula was occluded by transvenous coil embolization via the left internal jugular vein, and her symptoms resolved (Fig. 2A and B). One year after the treatment, the patient presented with tinnitus on the left, and diagnostic angiography showed a left ACC and transverse-sigmoid sinus (TSS) DAVF fed mainly by the left ascending pharyngeal artery and middle meningeal artery and draining into the left internal jugular vein without dangerous cortical venous reflux (Fig. 2C and D). Therefore, we decided to wait until the DAVF developed an aggressive pattern with dangerous cortical venous reflux. Two years after the diagnosis, although the ACC and TSS DVAF did not develop dangerous cortical venous reflux, the patient presented with tinnitus on the left side. We decided to perform a transvenous embolization of the left ACC and TSS DAVF. One month after diagnostic selective cerebral angiography for treatment, control cerebral angiography showed that the ACC and TSS DAVF had spontaneously occluded and her symptoms fully recovered (Fig. 2D).
DAVF is a pathological connection between the meningeal arteries and venous sinuses without an intervening nidus; the most common locations for DAVFs are the CS and TSS. Although the pathophysiology of DAVF development remains unclear, lesions may be acquired after head trauma, venous outflow obstruction; or venous hypertension, as in venous thrombosis. Generally, DAVF is treated by the disconnection of venous drainage via transarterial or transvenous procedures [10].
Recent studies have reported that 7–8% of patients with intracranial DAVFs have synchronous multiple DAVFs [2]. As shown in Table 1, previous reports, including the present cases, show that nine of the 11 de novo DAVFs developed in the regions downstream from the venous system after treatment of the previously discovered DAVFs. However, it is extremely rare for a DAVF to develop in a different location after treatment for the initial DAVF. Only one study reported a case of de novo DAVF in the ACC after transvenous embolization for a contralateral ACC DVAF [3]. At our institute, we performed transarterial or transvenous embolization for 104 cases of intracranial DAVFs between January 2012 and December 2020, and among these, two cases DAVFs developed de novo DAVFs. Case 1 was a de novo DAVF that occurred in the SLSW 4 years after transvenous embolization for AVF in another location; and DAVFs in the SLWS are rarely reported. The mechanism of de novo DAVF is considered to be vascular inflammation, angiogenesis, or thrombosis due to manipulation of the microcatheter or microguidewire of the venous or arterial wall. Moreover, venous hypertension or venous turbulence after treatment for shunt point disconnection may result in secondary DAVFs [5]. Ha et al. [2] demonstrated that 71.4% of patients with multiple DAVFs had cerebral venous sinus thrombosis, which can promote new channels between arteries and veins. In our cases, no signs of sinus thrombosis or sinus stenosis were detected. Additionally, we considered that shunt occlusion of the initial DAVF may have lead to alternative arterial flow into other sinus lesions, causing the de novo DAVFs. However, cerebral angiography and arterial spin labeling perfusion MR after treatment for the initial DAVFs did not show clear hemodynamic changes. Therefore, the DAVFs may have developed in association with unknown factors.
Previous reports have indicated that development of DAVFs in other locations might require a few months after transvenous or transarterial embolization [1,4,6,7,11]. Table 1 shows that the interval time from the treatment of one DAVFs to the diagnosis of de novo DAVFs was 4–60 months. Interestingly, ipsilateral de novo DAVFs develop within 1 year (mean, 6.7 months), whereas the interval between treatment of initial lesions and development of contralateral de novo DAVFs tends to be longer (mean, 43.6 months). Generally, angiographic examinations, such as MRI or cerebral angiography, are conducted 2 or 3 years after treatment for DAVFs. It is important to recognize that further clinical and angiographic follow-up may be required to detect de novo DAVFs.
De novo DAVF, though rare, can develop at remote sites following treatment for an initially diagnosed and treated DAVF. The appearance of contralateral de novo DAVFs in particular trends to take a longer time. Therefore, we need to evaluate the long-term angiographic follow-up of patients with DAVFs treated with endovascular embolization to detect de novo DAVFs.
Notes
Informed consent
Informed consent was obtained from all individual participants included in this study.
References
1. Gupta R, Horowitz M, Tayal A, Jovin T. De novo development of a remote arteriovenous fistula following transarterial embolization of a carotid cavernous fistula: case report and review of the literature. AJNR Am J Neuroradiol. 26:2587–2590. 2005.
2. Ha SY, Kwon YS, Kim BM, Kim DI, Kim DJ. Clinical and angiographic characteristics of multiple dural arteriovenous shunts. AJNR Am J Neuroradiol. 33:1691–1695. 2012.

3. Hagiwara S, Miyazaki T, Tsuji M, Kambara M, Yoshikane T, Nagai H, et al. A case of de novo anterior condylar dural arteriovenous fistula long after curative transvenous embolization of contralateral anterior condylar arteriovenous fistula. Case Rep Med. 2016:6974526. 2016.

4. Kiyosue H, Tanoue S, Okahara M, Yamashita M, Nagatomi H, Mori H. Recurrence of dural arteriovenous fistula in another location after selective transvenous coil embolization: report of two cases. AJNR Am J Neuroradiol. 23:689–692. 2002.
5. Kubota Y, Ueda T, Kaku Y, Sakai N. Development of a dural arteriovenous fistula around the jugular valve after transvenous embolization of cavernous dural arteriovenous fistula. Surg Neurol. 51:174–176. 1999.

6. Kurl S, Vanninen R, Saari T, Hernesniemi J. Development of right transverse sinus dural arteriovenous malformation after embolisation of a similar lesion on the left. Neuroradiology. 38:386–388. 1996.

7. Makiuchi T, Takasaki K, Yamagami M, Oda H, Todoroki K, Atsuchi M, et al. A case of sigmoid sinus dural arteriovenous fistula after treated cavernous dural arteriovenous fistula. Interv Neuroradiol 4 Suppl. 1:219–222. 1998.

8. Nakagawa H, Kubo S, Nakajima Y, Izumoto S, Fujita T. Shifting of dural arteriovenous malformation from the cavernous sinus to the sigmoid sinus to the transverse sinus after transvenous embolization. A case of left spontaneous carotid-cavernous sinus fistula. Surg Neurol. 37:30–38. 1992.

9. Newton TH, Cronqvist S. Involvement of dural arteries in intracranial arteriovenous malformations. Radiology. 93:1071–1078. 1969.

10. Oh JS, Yoon SM, Oh HJ, Shim JJ, Bae HG, Lee KS. Endovascular treatment of dural arteriovenous fistulas: single center experience. J Korean Neurosurg Soc. 59:17–25. 2016.

11. Yamashita K, Taki W, Nakahara I, Nishi S, Sadato A, Kikuchi H. Development of sigmoid dural arteriovenous fistulas after transvenous embolization of cavernous dural arteriovenous fistulas. AJNR Am J Neuroradiol. 14:1106–1108. 1993.
Fig. 1.
Right ascending pharyngeal artery angiogram demonstrates a left anterior condylar confluence dural arteriovenous fistula (DAVF) draining to left internal jugular and vertebral venous plexus (A, arrow). Postoperative cerebral angiogram demonstrates obliteration of the DAVF (B). Three-dimensional digital subtraction angiogram demonstrates that the DAVF (asterisk) around the sinus of the lesser sphenoid wing (SLSW) is mainly fed by the accessory meningeal artery. The DAVF drains from the SLWS through the sphenopetrosal vein and superior petrosal sinus into the inferior petrosal sinus via the cavernous sinus (dotted arrow) (C). The maximum intensity projection image on cerebral angiogram shows that retrograde leptomeningeal venous drainage is refluxed from the SLSW (white arrowhead) to the superficial middle cerebral vein (double arrowhead) via a bridging vein (white arrow) (D). Postoperative cerebral angiogram shows complete remission of the DAVF (E). SMCV : superficial middle cerebral vein, CS : cavernous sinus, SPV : sphenopetrosal vein, SPS : superior petrosal sinus, AMA : accessory meningeal artery, IPS : inferior petrosal sinus.
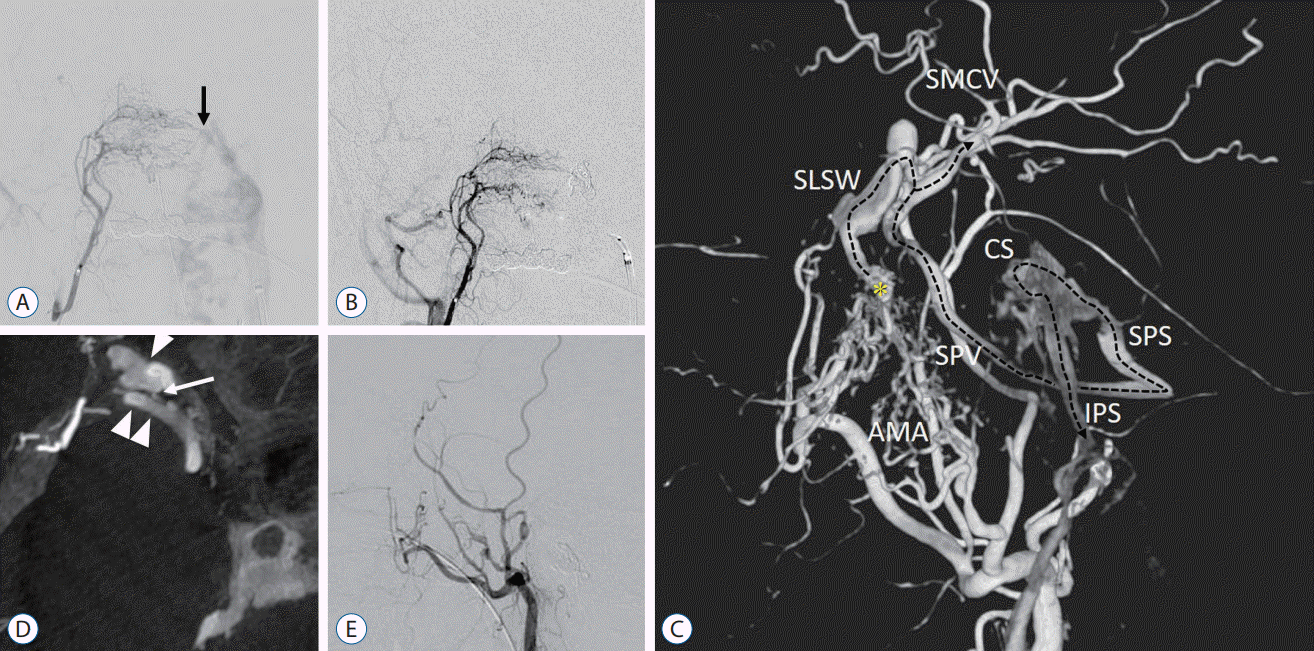
Fig. 2.
Cerebral angiogram demonstrates that the left cavernous sinus dural arteriovenous fistula (DAVF) (asterisk) drains to the superficial middle cerebral vein (black arrow) (A). Postoperative cerebral angiogram shows that the fistula is occluded (B). Three-dimensional digital subtraction angiogram shows that the left anterior condylar confluence and transverse-sigmoid sinus (asterisks) DAVFs are mainly fed by the left ascending pharyngeal artery (APA) and middle meningeal artery (MMA) and drain to the left internal jugular vein without dangerous cortical venous reflux (dotted arrow) (C). Cerebral angiogram shows that the DAVFs are spontaneously are occluded (D). TSS : transverse-sigmoid sinus.
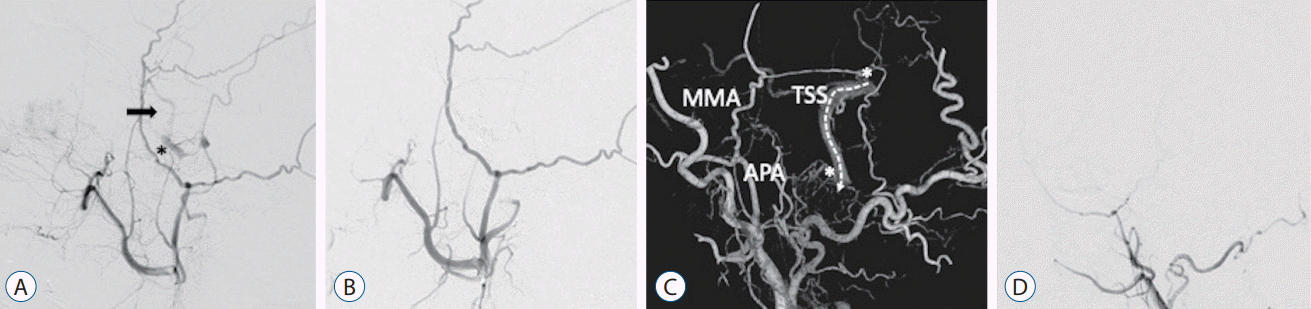
Table 1.
Summary of case reports of de novo dural arteriovenous fistula after endovascular embolization of dural arteriovenous fistula
| Study | Age/sex | Initial location | Treatment | Time interval (months) | De novo location |
|---|---|---|---|---|---|
| Gupta et al. [1] (2005) | 59/F | Left CS | TAE | 4 | Left SS |
| Hagiwara et al. [3] (2016) | 55/M | Right ACC | TVE | 60 | Left jugular foramen |
| Kiyosue et al. [4] (2002) | 62/M | Left CS | TVE | 5 | Left SS |
| Kiyosue et al. [4] (2002) | 66/F | Left CS | TVE | 5 | Left JB |
| Kubota et al. [5] (1999) | 43/F | Left CS | TVE | 4 | Left JB |
| Kurl et al. [6] (1996) | 54/F | Left TSS | TAE | 23 | Right TSS |
| Makiuchi et al. [7] (1998) | 43/M | Left CS | TVE | 6 | Left SS |
| Nakagawa et al. [8] (1992) | 43/F | Left CS | TAE | 6 | Left TS |
| Yamashita et al. [11] (1993) | 54/F | Right CS | TAE | 12 | Right TSS |
| Present case 1 | 72/F | Left ACC | TVE | 48 | Right SLSW |
| Present case 2 | 69/F | Left CS | TVE | 12 | Left ACC |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download