This article has been
cited by other articles in ScienceCentral.
Abstract
Kawasaki disease shock syndrome (KDSS) is defined as a sustained decrease in systolic blood pressure or signs of poor perfusion. Some children with KDSS are refractory to conventional therapy, such as intravenous immunoglobulin (IVIG). A 4-year-old boy with Kawasaki disease was referred to the emergency department. Despite the conventional therapy, his vital signs and cardiac function worsened. The boy promptly underwent extracorporeal membrane oxygenation (ECMO), which did not relieve the symptoms. Subsequently, he underwent methylprednisolone pulse therapy and the second cycle of IVIG therapy. Two days after the start of a combination of ECMO, steroids, and IVIG started, his clinical condition was stabilized, and finally, ECMO was removed. This case highlights the combination of ECMO, steroids, and IVIG as a treatment option for children with IVIG-resistant KDSS.
Go to :

Keywords: Extracorporeal Membrane Oxygenation, Immunoglobulins, Intravenous, Mucocutaneous Lymph Node Syndrome, Shock, Cardiogenic, Steroids
Introduction
Kawasaki disease shock syndrome (KDSS), a rare complication of Kawasaki disease (KD), is defined as a sustained decrease in systolic blood pressure (BP) from a baseline of ≥ 20% or signs of poor perfusion
1). Pathophysiology of KDSS may be related to vasculitis with capillary leakage and high concentrations of multiple cytokines
2). To reduce systemic and vascular inflammation, treatment options may include intravenous immunoglobulin (IVIG), steroids or tumor necrosis factor-α blocker
3). However, some children rapidly deteriorate, and are refractory to the conventional therapy. We report a case of IVIG-resistant KDSS rescued by a combination of extracorporeal membrane oxygenation (ECMO), steroids, and IVIG.
Go to :

Case
A previously healthy 4-year-old boy was referred to the emergency department (ED) for 5-day history of fever, swelling of bilateral cervical lymph nodes, and abdominal pain. One day after the onset, the symptoms were accompanied by multiple irregular-shaped skin lesions around the neck and chest, bilateral conjunctival injection, and erythema on the lips. Ampicillin-sulbactam and cefotaxime had been administered for 2 days until the arrival to the ED.
The initial vital signs were as follows: BP, 81/41 mmHg (ninth and 19th percentiles, respectively); heart rate, 149 beats/minute; respiratory rate, 35 breaths/minute; temperature, 38.5°C; and oxygen saturation, 97% on room air. On physical examination, the boy was lethargic and had cold extremities and skin mottling. Laboratory findings were as follows: white blood cells, 23,520/mm³; hemoglobin, 9.4 g/dL; aspartate aminotransferase, 71 U/L; alanine aminotransferase, 175 U/L; myoglobin, 15 ng/mL (reference value, 12-80 ng/mL); creatine kinase-myocardial band, 8.2 ng/mL (reference value, 0.5-3.1 ng/mL); troponin-I, 0.07 ng/mL (reference value, 0-0.04 ng/mL); brain natriuretic peptide, > 4,988 pg/mL (reference value, 23-327 pg/mL); natrium, 133 mmol/L; and C-reactive protein, 15.7 mg/dL. Transthoracic echocardiography (TTE) showed a 50% ejection fraction (EF) with a 3.0 mm diameter of the distal right coronary artery (RCA) (Z-score = 3.1). He was hospitalized to the intensive care unit, and was immediately administered 2 g/kg of IVIG (day 1).
Twelve hours later (day 2), his BP rapidly dropped to 28/22 mmHg and the EF decreased to 20%. Although dopamine and dobutamine (both, 20 μg/kg/min) and epinephrine (0.5 μg/kg/min) were infused, BP did not recover. We placed a central veno-arterial (VA) ECMO on the boy with a centrifugal pump and an artificial lung. The right common carotid artery and internal jugular vein were cannulated (
Fig. 1). Although he had been supported on ECMO for 14 hours with infusion of epinephrine (1 μg/kg/min) and norepinephrine (0.4 μg/kg/min), BP remained 40-53/34-42 mmHg and the enzymes were as follows: myoglobin, 300 ng/mL; creatine kinase-myocardial band, 9.8 ng/mL; and troponin-I, 0.41 ng/mL. TTE showed a 20% EF with a 3.2 mm diameter of the distal RCA (Z-score = 3.6). Thus, 21 hours after the first administration (day 2), we decided to immediately start a methylprednisolone pulse therapy and the second cycle of IVIG with maintenance of the ECMO, steroids, and inotropes. BP was normalized in 24 hours (day 3; range, 75-84/61-72 mmHg) and the inotropes were gradually tapered. On day 4, the vital sign was stabilized with a 41% EF and the ECMO was successfully removed from the boy.
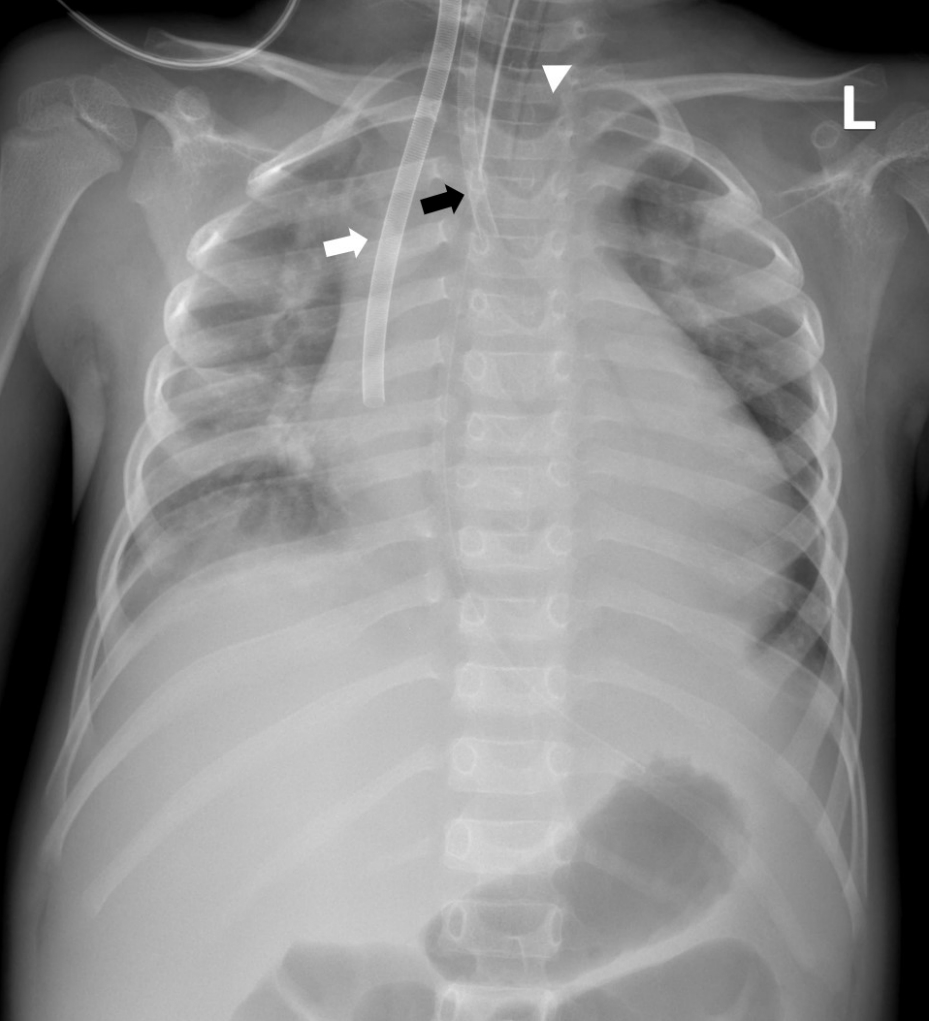 | Fig. 1.The chest radiograph obtained just after the commencement of extracorporeal membrane oxygenation on day 2. The radiograph shows cardiomegaly, increased pulmonary vascularity, and multiple subsegmental atelectases. Note the cannulae inserted in the right internal jugular vein (15-French, white arrow) and carotid artery (12-French, black arrow) with the endotracheal tube inserted (arrowhead). 
|
A follow-up TTE on day 24 demonstrated an aneurysm of the distal RCA with a 53.8% EF (
Fig. 2). The echocardiogram also showed another fusiform aneurysm at the proximal RCA (diameter, 5.2 mm; Z-score = 8.0) with a normal finding of the left anterior descending coronary artery. On day 29, the boy was discharged on aspirin, clopidogrel, and warfarin to prevent coronary thrombosis.
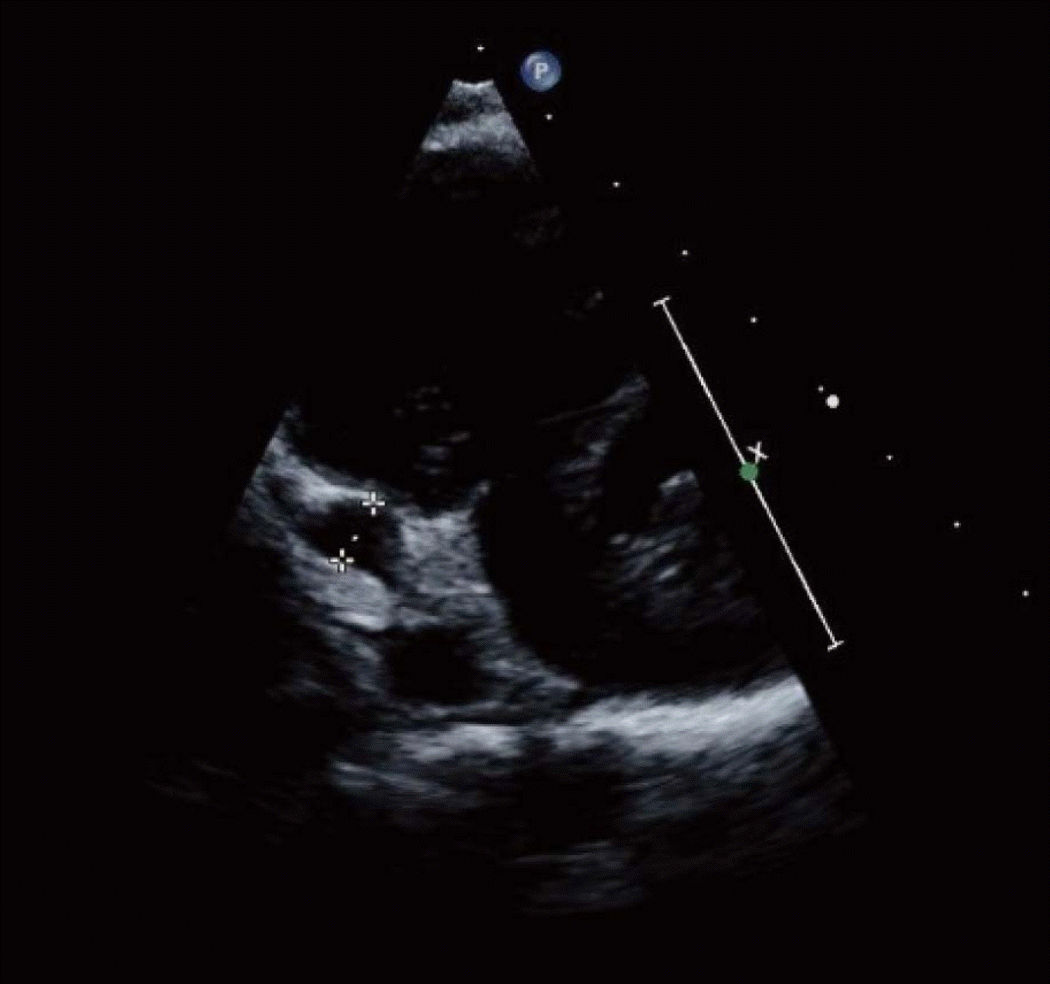 | Fig. 2.Echocardiogram on day 24. It shows a fusiform teardrop-shaped aneurysm of the distal right coronary artery (dotted caliper; diameter, 6.7 mm; Z-score = 13.5). 
|
Go to :

Discussion
In KD, IVIG and aspirin comprise conventional therapy and its fatality rate is lower than 1%. However, it could develop into fatal KDSS
4,
5). A recent study showed more atypical features of KD and a lower rate of initial diagnosis of KD in KDSS group than in KD group (23.1% vs. 80.2%)
6). Compared with children with KD, those with KDSS were more refractory to the first cycle of IVIG (76.9% vs. 19.8%), and more frequently required further treatment, such as methylprednisolone pulse therapy (38.5% vs. 11.0%) and infliximab (15.4% vs. 0%)
6).
To date, a few studies have reported about IVIG-resistant KDSS. Although there is a debate on IVIG and steroids as the primary treatment of KD, steroids are usually recommended for those who are refractory to IVIG or have severe inflammation
3). For example, 3 children with IVIG-resistant KDSS were successfully treated with steroid pulse therapy
7). We noted 2 cases describing a total of 3 children with KDSS who underwent VA ECMO
8,
9). The 3 children survived with help of VA ECMO and IVIG although the administration of IVIG and fluids was delayed due to their manifestations mimicking toxic shock syndrome or septic shock. Zhang et al.
8) emphasized the early diagnosis of KDSS and administration of IVIG. In addition, they suggested VA ECMO as a good treatment option for children with KDSS who are in need of cardiac support
8).
This case describes a boy with IVIG-resistant KDSS who was rescued by the combination of ECMO, steroids, and IVIG. In EDs, KDSS is suggested by unexplained fever and cardiac dysfunction. The combination could be a treatment option for children with KDSS refractory to the conventional therapy.
Go to :






 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download