Abstract
Background
Patients who undergo coronary artery bypass graft (CABG) surgery receive regular physical examinations and medications on an outpatient basis. However, these patients are at risk of developing other vascular diseases, such as postoperative arterial steno-occlusive disease (SOD). This study investigated the incidence of SOD and related factors.
Methods
In total, 246 patients who underwent CABG surgery from January 1, 2017 to December 31, 2021 were investigated. The incidence and risk factors of vascular disease were analyzed by dividing the included patients into SOD and non-SOD groups. Laboratory tests, medical history, surgical information, and family history were investigated through an electronic chart review.
Results
Data from 193 patients who met the criteria were analyzed. SOD occurred in 19.1% of patients, and the cerebral artery (38%) was the most common artery involved, followed by the peripheral artery (32%), the coronary artery (22%), and the retinal artery (8%). Risk factors for the development of SOD included estimated glomerular filtration rate (eGFR; odds ratio [OR]=0.977, p=0.008), cholesterol (OR=1.020, p=0.001), and patients with diabetes complications (OR=5.077, p=0.010). The 3-year cumulative incidence rate was 21.6%, and the risk factors for cumulative occurrence were a low eGFR, elevated cholesterol, and complications of diabetes.
Coronary artery bypass graft (CABG) surgery and percutaneous coronary intervention are two different therapeutic approaches for treating coronary artery disease (CAD) [1-4]. Surgery is usually performed in patients with diseases that involve multiple coronary arteries [5]. CAD is usually caused by atherosclerosis, a buildup of plaque inside the artery walls, which can either affect a single or more blood vessels throughout the body [6]. Although these patients might already have small artery stenosis or occlusion at the time of surgery, a significant portion of patients could be diagnosed with newly developed arterial disease after surgery.
After surgery, follow-up is mainly performed on an outpatient basis through medications, blood tests, and physical and radiologic examinations. Arterial disease can have a significant impact on patients and affect their quality of life and can have various clinical manifestations from claudication to stroke and even myocardial infarction.
The prevalence of other vascular diseases found in preoperative examinations has been reported in a number of studies. However, there are few reports on the frequency and risk factors of small artery disease in follow-up of patients after surgery. The purpose of this study was to investigate the frequency and factors related to postoperative arterial steno-occlusive disease (SOD).
Ethics statements: This study was approved by the Institutional Review Board of the Daegu Catholic University Hospital (IRB No. CR-22-020) with exemption from informed consent. Personal data related to patients’ information were used, and personally identifiable information was protected. This study was a retrospective observational study, and this manuscript did not contain personally identifiable information. In addition, all study methods were performed in compliance with relevant guidelines and regulations.
This study included patients who received CABG for CAD from Daegu Catholic University Medical Center from January 1, 2017 to December 31, 2021. Patients who were already suffering from vascular disease at the time of surgery, cases with follow-up loss, or death after surgery were excluded. Data were collected retrospectively, and the patients’ medical records were used. We investigated sex, age, and medical history as the baseline characteristics. The type of surgery, the number of anastomosed arterial vessels, and blood test results were also collected. Patients with vascular SOD were selected according to their outpatient treatment. All included patients were distinguished into two groups, namely, SOD and non-SOD groups, and statistical analysis was performed.
Vascular SOD was defined as a disease group requiring treatment due to symptoms. Cerebrovascular accidents include retinal artery occlusion, peripheral arterial occlusive disease, and disease of native coronary artery or grafted vessels. If examination was necessary, computed tomography, magnetic resonance imaging, and angiograms were performed at the department in charge.
Patients complaining of decreased visual acuity and decreased visual field after surgery were referred to the ophthalmologist in this hospital for a fundus examination. At that time, patients had their best corrected visual acuity and intraocular pressure assessed by the ophthalmologist, who then performed a dilated fundus examination. In cases of suspected retinal vascular occlusive disease, fluorescein angiography was additionally performed to confirm the diagnosis. Patients diagnosed with retinal artery occlusion (central and branch) or ocular ischemic syndrome were included in the study.
CABG surgery was performed by median sternotomy after general anesthesia. Off-pump CABG was performed only in cases where aortic cross clamp was difficult to implement due to severe aortic atherosclerosis. On-pump CABG was performed to the remaining patients. Since the conduit was used as a graft, the left internal mammary artery was used as the basis, and great saphenous vein harvesting was performed according to the requirement for additional sites of anastomosis. After surgery, all patients received antiplatelet therapy. After discharge, follow-up was performed at the outpatient clinic every 3–6 months.
Categorical data are expressed as frequencies and percentages, and continuous data are expressed as mean±standard deviations with ranges. The chi-square test or Fisher exact test was used for categorical data, and the Mann-Whitney test was used for continuous data. Logistic regression analysis was performed to determine the risk factor for SOD. Kaplan-Meier was used to calculate the cumulative incidence. Cox regression analysis was performed to identify the risk factors of the cumulative incidence rate.
Of the 246 patients initially included, we excluded 53 patients who did not visit the outpatient department within the last 6 months or had already suffered non-coronary artery SOD at the time of surgery.
The basic patient characteristics included in this study are summarized in Table 1. Of a total of 193 patients, 37 patients (19.1%) suffered SOD, and the detailed ratio is shown in Fig. 1. The mean age of the non-disease and SOD groups was 65.7 and 65.2 years, respectively. The proportions of males were 76.3% and 75.7%, respectively, whereas smoking and medical disease history rates were similar in both groups.
Table 2 demonstrates the operative and laboratory data. In both groups, conventional CABG was mainly performed, and the most common number of anastomoses required was three. Although there was no difference in most laboratory findings between the two groups, the estimated glomerular filtration rate (eGFR) was slightly higher in the non-SOD group, whereas cholesterol was higher in the SOD group. However, the differences were not statistically significant.
Table 3 shows the results of the risk factor analysis for SOD occurrence through multivariate logistic regression analysis. When there was a history of diabetes mellitus (DM) complications, the risk was approximately five times higher, and the p-value was 0.01. The higher the cholesterol, the higher the risk, and the higher the eGFR, the lower the risk. Patients with a family history of vascular disease had an approximately 2.7-fold increased risk, but there was no statistical significance.
The cumulative incidence rate is shown in Fig. 2. The overall 3-year cumulative incidence rate was 21.6%, but this rate increased to 89.2% in the SOD group. Risk factors for the cumulative occurrence of SOD included eGFR, elevated cholesterol, and history of DM complications.
Among the causes of SOD, non-atherosclerosis such as vasculitis, Buerger disease, and fibromuscular dysplasia are also known [7-9]. However, none of the patients included in our study were diagnosed with any of the above diseases. It is thought to be mainly caused by atherosclerosis. Atherosclerosis is a systemic disease associated with inflammation [10,11].
The majority of patients treated with CABG suffer from multivascular coronary artery stenosis or occlusion. In addition to the coronary arteries, these patients are likely to suffer from similar arterial disease throughout the body.
The prevalence of arterial disease at the time of CABG surgery has been previously reported in the literature [12,13]. In particular, carotid artery stenosis has been detected in a significant number of patients during preoperative CABG examinations, and thus, it is controversial whether to perform simultaneous or staged operation [14,15].
Nonetheless, the incidence of vascular disease after surgery has not been reported in the literature. In our study, we investigated the pattern of disease development in other blood vessels throughout the body in patients who had already developed CAD and had undergone CABG surgery.
In this study, patients’ data were followed for a period of 5 years after CABG surgery. Among them, 19.1% of patients developed SOD after surgery. Head vessels were the most common, followed by peripheral arterial occlusive disease and coronary artery. One issue that might influence the incidence of these findings is that the examinations started and a positive diagnosis was reached as soon as the patient started to complain of symptoms related to vascular disease.
In our study, we found that the risk factors that may influence the development of new vascular SOD after surgery in CABG patients were low eGFR and complications of DM, high cholesterol, and a family history of vascular disease.
To our knowledge, this is the first study providing results from patients who have undergone CABG. Therefore, commonly known risk factors for SOD of blood vessels are listed. Risk factors for cerebral artery disease are hypertension, smoking, DM, and hyperlipidemia [16-19], whereas risk factors for peripheral arterial disease include DM, hyperlipidemia, arterial hypertension, platelet activity, and smoking [20-22]. Our study reveals that the most important concerns for CABG patients is the onset of symptoms due to graft patency problems or native vessel disease progression after surgery. Risk factors for these important events include sex, hypertension, hyperlipidemia, DM, and chronic kidney disease [23-25].
The overall 3-year cumulative incidence rate was 21.6%, and the risk factors for the cumulative incidence were low eGFR, high cholesterol, and DM complications.
According to this study, a significant number of patients who received CABG may suffer from SOD in other arteries. In particular, patients with low eGFR, patients with DM complications, and patients with poor cholesterol control may develop vascular disease. Therefore, it is necessary to assess the patients’ kidney function and cholesterol levels through periodic blood tests at outpatient clinics. Patients with high cholesterol should be prescribed with a lipid-lowering agent, whereas patients with poor cholesterol control should be referred to a related internal medicine clinic to receive appropriate treatment.
A limitation of this study is that it is a retrospective single-center study. Furthermore, there was no detailed patient information regarding any concomitant use of medications, such as antiplatelet drugs or hyperlipidemia drugs, during this study. Therefore, it is unknown how or whether these medications have affected the clinical results.
In conclusion, patients with low eGFR, high cholesterol, or diabetic complications, who have previously undergone CABG surgery, are particularly at increased risk of developing other vascular diseases. Therefore, it is necessary for these patients to receive the appropriate medication in the outpatient department and to have a close referral system with the relevant department.
Notes
Author contributions
Conceptualization: GWL. Data curation: SGK. Formal analysis: CHL. Investigation: SGK. Methodology: GWL, CHL. Project administration: CHL. Resources: GWL. Software: CHL. Supervision: CHL. Validation: CHL. Visualization: SGK, LGW, CHL. Writing - original draft: SGK. Writing - review & editing: CHL.
References
1. Bypass Angioplasty Revascularization Investigation (BARI) Investigators. Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease. N Engl J Med. 1996; 335:217–25.
2. BARI 2D Study Group, Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009; 360:2503–15.

3. King SB 3rd, Lembo NJ, Weintraub WS, Kosinski AS, Barnhart HX, Kutner MH, et al. A randomized trial comparing coronary angioplasty with coronary bypass surgery. Emory Angioplasty versus Surgery Trial (EAST). N Engl J Med. 1994; 331:1044–50.

4. Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009; 360:961–72.

5. Farkouh ME, Domanski M, Sleeper LA, Siami FS, Dangas G, Mack M, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012; 367:2375–84.

7. Saadoun D, Vautier M, Cacoub P. Medium- and large-vessel vasculitis. Circulation. 2021; 143:267–82.

9. Gornik HL, Persu A, Adlam D, Aparicio LS, Azizi M, Boulanger M, et al. First International Consensus on the diagnosis and management of fibromuscular dysplasia. Vasc Med. 2019; 24:164–89.

10. Anogeianaki A, Angelucci D, Cianchetti E, D'Alessandro M, Maccauro G, Saggini A, et al. Atherosclerosis: a classic inflammatory disease. Int J Immunopathol Pharmacol. 2011; 24:817–25.

11. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005; 352:1685–95.

12. Wanamaker KM, Moraca RJ, Nitzberg D, Magovern GJ Jr. Contemporary incidence and risk factors for carotid artery disease in patients referred for coronary artery bypass surgery. J Cardiothorac Surg. 2012; 7:78.

13. Huang CH, Hsu CP, Lai ST, Weng ZC, Tsao NW, Tsai TH. Operative results of coronary artery bypass grafting in women. Int J Cardiol. 2004; 94:61–6.

14. Drohomirecka A, Koltowski L, Kwinecki P, Wronecki K, Cichon R. Risk factors for carotid artery disease in patients scheduled for coronary artery bypass grafting. Kardiol Pol. 2010; 68:789–94.
15. Venkatachalam S, Gray BH, Mukherjee D, Shishehbor MH. Contemporary management of concomitant carotid and coronary artery disease. Heart. 2011; 97:175–80.

16. Resch JA, Baker AB. Etiologic mechanisms in cerebral atherosclerosis: preliminary study of 3,839 cases. Arch Neurol. 1964; 10:617–28.
17. Sacco RL, Kargman DE, Zamanillo MC. Race-ethnic differences in stroke risk factors among hospitalized patients with cerebral infarction: the Northern Manhattan Stroke Study. Neurology. 1995; 45:659–63.

18. Ingall TJ, Homer D, Baker HL Jr, Kottke BA, O'Fallon WM, Whisnant JP. Predictors of intracranial carotid artery atherosclerosis: duration of cigarette smoking and hypertension are more powerful than serum lipid levels. Arch Neurol. 1991; 48:687–91.

19. Rincon F, Sacco RL, Kranwinkel G, Xu Q, Paik MC, Boden-Albala B, et al. Incidence and risk factors of intracranial atherosclerotic stroke: the Northern Manhattan Stroke Study. Cerebrovasc Dis. 2009; 28:65–71.

20. Allison MA, Criqui MH, McClelland RL, Scott JM, McDermott MM, Liu K, et al. The effect of novel cardiovascular risk factors on the ethnic-specific odds for peripheral arterial disease in the Multi-Ethnic Study of Atherosclerosis (MESA). J Am Coll Cardiol. 2006; 48:1190–7.

21. Murabito JM, D'Agostino RB, Silbershatz H, Wilson WF. Intermittent claudication: a risk profile from the Framingham Heart Study. Circulation. 1997; 96:44–9.
22. Lu Y, Ballew SH, Tanaka H, Szklo M, Heiss G, Coresh J, et al. 2017 ACC/AHA blood pressure classification and incident peripheral artery disease: the Atherosclerosis Risk in Communities (ARIC) Study. Eur J Prev Cardiol. 2020; 27:51–9.

23. McLean RC, Nazarian SM, Gluckman TJ, Schulman SP, Thiemann DR, Shapiro EP, et al. Relative importance of patient, procedural and anatomic risk factors for early vein graft thrombosis after coronary artery bypass graft surgery. J Cardiovasc Surg (Torino). 2011; 52:877–85.
Fig. 1.
The distribution of involved sites in cases of steno-occlusive disease in our study. Cerebral artery disease and peripheral artery disease had the highest rates (38% and 32%, respectively). Coronary artery disease involving the graft and native vessels occurred in 22% of cases. Retinal artery disease demonstrated the lowest rate (8%).
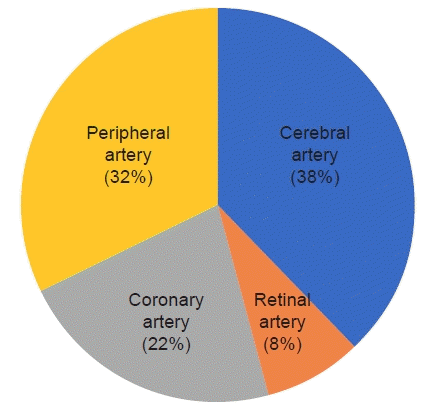
Fig. 2.
The cumulative incidence of steno-occlusive disease (SOD) after coronary artery bypass graft (CABG). This figure shows the cumulative incidence of SOD in the study group. The overall 3-year cumulative incidence rate was 21.6%.
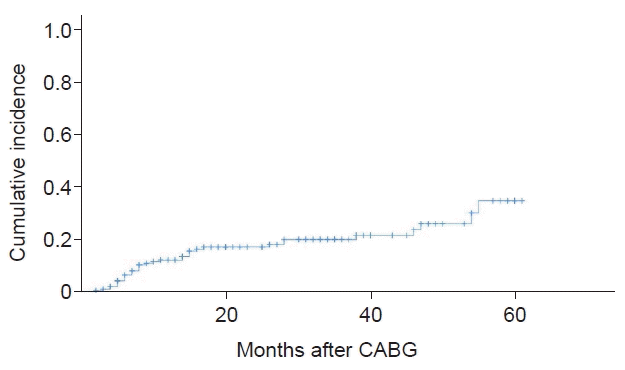
Table 1.
Basic characteristics of the two groups
Table 2.
Comparison of clinical characteristics before and during surgery between the two groups




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download