Abstract
High pre-transplant isoagglutinin is a risk factor for antibody-mediated rejection in ABO-incompatible living donor liver transplantation. A 55-year-old man with alcoholic liver cirrhosis underwent ABO-incompatible living donor liver transplantation. The initial isoagglutinin immunoglobulin G titer was 1:1,024. Despite five sessions of plasmapheresis, the isoagglutinin titer was not significantly reduced (from 1:1,024 to 1:512). We decided to perform 11 plasmaphereses and proceed with liver transplantation regardless of the isoagglutinin titer (1:128 at transplantation day). Instead, we planned to administer 0.5 g/kg intravenous immunoglobulin and booster rituximab (200 mg) after transplant. On postoperative day 6, the isoagglutinin titer increased from 1:32 to 1:64, and the patient received plasmapheresis twice. The patient maintained stable liver function without evidence of further complications or rejection. The high-dose intravenous immunoglobulin, salvage plasmapheresis, and booster rituximab protocol might be able to overcome a pre-transplant high isoagglutinin titer in ABO-incompatible living donor liver transplantation without splenectomy.
The incompatibility of ABO blood type between donor and recipient is one of the significant barriers to liver transplantation (LT) [1]. Many transplant centers have proposed Various desensitization protocols to overcome the barrier of ABO incompatibility [2]. Plasmapheresis (PP), splenectomy, intravenous immunoglobulin (IVIG) administration, aggressive immunosuppressive protocols, and intrahepatic portal and arterial infusions of anti-inflammatory drugs, such as rituximab, have all been utilized to improve the outcome of ABO-incompatible (ABOi) living donor liver transplant (LDLT) [3]. These strategies have three main objectives: (1) suppression of B-cell activity, (2) attenuation of local inflammation, and (3) pre-transplant reduction of isoagglutinin (IA) titers. Since the introduction of rituximab, the graft survival of ABOi-LDLT has improved dramatically by the depletion of CD20 positive B cells [4]. Moreover, the effectiveness of local graft infusion therapy with anti-inflammatory drugs such as prostaglandin E1, methylprednisolone, and gabexate mesylate is no longer effective in the era of rituximab [5]. Meanwhile, the role of pre-transplant ABO antibody titer in antibody-mediated rejection (AMR) is controversial in ABOi-LDLT [4]. Although the strategy to reduce IA titers has been considered to play a critical role in the success of ABOi-LDLT, recent studies proposed a simplified protocol without PP. However, those studies only included patients with a low baseline IA titer [2]. There is no established protocol or cutoff value for patients with a high titer or who do not respond to PP before LT. Here, we report a successful ABOi-LDLT in a recipient with high pre-transplant IA titer using our modified protocol.
Ethical statements: This study was approved by the Institutional Ethics Committee of Kosin University Gospel Hospital (2022-03-005). The informed consent was waived because this design is a retrospective study.
The patient was a 55-year-old man with alcoholic liver cirrhosis. He underwent a transjugular intrahepatic portosystemic shunt procedure due to recurrent episodes of esophageal variceal bleeding. Three months later, the patient underwent gastric varix embolization because of gastric variceal bleeding. Since then, endoscopic sclerotherapy has been performed because of gastric varix. Subsequently, ascites gradually developed, and his son donated his liver. His son's blood type (B+) was not compatible with the patient's (O+); therefore, we planned ABOi-LDLT. The patient's Child-Pugh score and model for end-stage liver disease score were C11 and 24, respectively. Initial titers of immunoglobulin G and immunoglobulin M were 1:1,054 and 1:256, respectively.
We administered 633 mg of rituximab (375 mg/m2 body surface area [BSA]) 3 weeks before the scheduled transplantation date, and the patient had no specific adverse reaction. We planned five sessions of PP with 5% albumin and two sessions of PP with fresh frozen plasma (FFP) as per our standard ABOi-LDLT protocol (Fig. 1). The patient's estimated plasma volume was 3,300 mL; 1.0 estimated plasma volume was exchanged as one session of PP. PP began a week after rituximab administration. Despite the 1st five PPs, the reduction in the IA titer was insignificant (from 1:1,024 to 1:512). We decided to postpone the LT and planned an additional PP. However, thrombocytopenia worsened after the 7th PP session, international normalized ratio increased to 3 or more, and bleeding occurred at the PP catheter insertion site. Therefore, the patient had to receive FFP transfusion, and it was determined that the IA titer goal of our protocol could not be reached. We decided to perform three more daily PPs with FFP and proceed with LT regardless of the IA titer. Instead, 0.5 g/kg IVIG from the anhepatic phase to postoperative day (POD) 5, booster rituximab 200 mg on POD 2, and salvage PP were planned after transplantation. The IA titer measured on the day of LT was 1:128 (Fig. 2).
The right liver (802 g) was donated, and the graft-to-recipient weight ratio was 1.20%. Two right inferior hepatic veins were made into one and connected to the inferior vena cava. V5 and V8, which are venous branches from the middle hepatic vein to the segments, were reconstructed using a cryopreserved iliac vein and anastomosed to the common trunk of the middle and left hepatic veins. The right anterior bile duct and right posterior bile duct were separated. Two bile openings were made into one hole and anastomosed to the common hepatic duct using an external bile stent.
On the 1st day after LT, bleeding around the adrenal gland was confirmed on computed tomography and did not resolve by intervention. Re-operation for bleeding control was performed via laparotomy. After this operation, the pulmonary edema became severe, and continuous renal replacement therapy was needed until POD 10.
We measured the cell count and percentage of CD19/20 B cells as markers serially before and after LT (Table 1). Before transplantation, the cell count and percentage of CD19/20 B cells were close to nadir. IA titer was maintained as 1:32 until POD 4. On POD 5, the IA titer increased to 1:64, and aspartate aminotransferase (AST)/alanine aminotransferase (ALT) rose more than twice as much as the previous day to 151/194. Therefore, two sessions of PPs were performed, IA titer decreased to 1:32, the AST/ALT was stabilized, and bile was well-drained through the external bile stent after that. The IA titer increased to 1:64 on POD 13, but we did not perform additional PPs because it was determined that graft was accommodated in the recipient and resistant to AMR. The total bilirubin level was 3.37 mg/dL on POD 1 and then steadily declined to 0.94 mg/dL on POD 29 at discharge. Cytomegalovirus polymerase chain reaction DNA was detected in the blood on POD 19; it was treated with ganciclovir, and no other infections occurred. Four months after the transplant, the external biliary stent was removed, the patient maintained stable liver function without evidence of infection, vascular or biliary complication, and rejection.
Pre-transplant high IA titer is a risk factor for postoperative AMR, biliary complications, and hepatic necrosis [6]. In most desensitization protocols, including pre-transplant PP, rituximab, IVIG, splenectomy, the pre-transplant IA titer target is at least 1:64 with or without PP. However, there is no established protocol in cases where the pre-transplant IA titer is very high, the titer does not fall enough after PP, or PP cannot be performed due to allergic reactions or other reasons. Several case reports have introduced their experiences and protocols in patients with pre-transplant high IA titers.
Lee et al. [4] reported three cases of successful ABOi-LDLT in patients with failed PP. They used a single dose of rituximab pre-transplant and post-transplant high-dose IVIG (0.8 g/kg from the anhepatic phase to 2 days after LT). They performed a splenectomy during LT to eradicate remnant antibody-producing plasma cells that PP cannot eliminate. Saitoh et al. [7] reported a very high pre-transplant IA titer of 1:4,096. They administered rituximab twice (500 mg of rituximab 2 weeks before LT and 300 mg of rituximab 1 day before LT), splenectomy, and pre-transplant and post-transplant PP without IVIG. Ikegami et al. [8] used high-dose IVIG (0.6 g/kg) and PP in a patient with rebound IA titer to 1:2,048 after LT with splenectomy.
In all reported cases, concomitant splenectomy was performed during LT, but splenectomy was not performed in our patient. Splenectomy in a patient with splenomegaly and developed collaterals takes a long operation time and carries the risk of massive bleeding [9]. In addition, splenectomy increases the risk of post-transplant infection and portal vein thrombosis, pancreatic fistula, and sepsis in patients undergoing heavy immunosuppression and PP [10]. Meanwhile, it is thought that rituximab can replace splenectomy as a chemical splenectomy. Rituximab has been proven to deplete CD20 positive B cells from the circulation and lymphoid tissues, including the spleen, within 72 hours [11]. However, despite the use of rituximab in the cases as mentioned above reports, the reason for splenectomy is to remove remnant antibody-producing plasma cells that cannot be eliminated by rituximab and PP [4].
Instead of splenectomy, we chose 200 mg of booster rituximab on POD 2 in this patient. B cells become activated only due to sensitization by ABO histo-group antigens on the vascular endothelial cells of the graft. Some B cells may escape rituximab depletion preoperatively and become activated B cells producing antibodies after transplantation [12]. Therefore, it may be beneficial that rituximab is administered postoperatively, especially in AMR-high risk patients, to deplete activated B cells completely.
In ABOi-LDLT, the most common strategy is to administer a single dose of rituximab (375 mg/m2 BSA or less) 1–4 weeks before ABOi-LDLT [13]. However, the optimal dosage and timing of rituximab in ABOi LT remains controversial. A 375 mg/m2 BSA dosage was adopted from lymphoma treatment protocols. Egawa et al. [14] considered that a dosage of <300 mg is probably insufficient. Furthermore, administering multiple doses of rituximab has been shown to increase the incidence of fungal infections and cytomegalovirus disease significantly [15].
IVIG is another immunologic strategy for ABOi-LDLT. The proposed mechanisms include the blockade of Fc receptors on mononuclear phagocytes, direct antibody neutralization, inhibition of CD19 expression on activated B cells, suppression of complement, and suppression of T cells [2]. We administered 0.5 g/kg/day of IVIG to this patient for 6 days from the anhepatic phase. A Japanese multicenter study reported that administration of IVIG did not decrease the incidence of AMR significantly [15]. However, IVIG and PP are used as rescue treatments in severe AMR [8]. IVIG might have worked on for B lymphocytes may have survived in the lymph node under rituximab therapy or may have been newly differentiated [16]. It is thought that further research on IVIG's optimal dose, timing, and frequency is needed.
The most critical factor for preventing AMR in recipients undergoing ABOi-LDLT is the suppression of de novo antibodies. If the baseline titer before LT is high, the titer does not respond to PP, or PP cannot be performed, the protocol of well-combined high-dose IVIG, salvage PP, booster rituximab without splenectomy might be considered to be able to overcome ABOi-LDLT.
References
1. Kim JM, Kwon CH, Joh JW, Kang ES, Park JB, Lee JH, et al. ABO-incompatible living donor liver transplantation is suitable in patients without ABO-matched donor. J Hepatol. 2013; 59:1215–22.

2. Oh J, Kim JM. Immunologic strategies and outcomes in ABO-incompatible living donor liver transplantation. Clin Mol Hepatol. 2020; 26:1–6.

3. Tanabe M, Kawachi S, Obara H, Shinoda M, Hibi T, Kitagawa Y, et al. Current progress in ABO-incompatible liver transplantation. Eur J Clin Invest. 2010; 40:943–9.

4. Lee B, Cho JY, Lee HW, Choi Y, Yoon YS, Han HS. Successful ABO-incompatible living donor liver transplantation using splenectomy and intravenous immunoglobulin in high isoagglutinin titer patients. Korean J Transplant. 2020; 34:109–13.

6. Kozaki K, Egawa H, Ueda M, Oike F, Yoshizawa A, Fukatsu A, et al. The role of apheresis therapy for ABO incompatible living donor liver transplantation: the Kyoto University experience. Ther Apher Dial. 2006; 10:441–8.

7. Saitoh Y, Fujio A, Miyagi S, Tokodai K, Unno M, Kamei T. ABO-incompatible living donor liver transplantation with high preoperative antibody titer: a case report. Int J Surg Case Rep. 2021; 85:106260.

8. Ikegami T, Taketomi A, Soejima Y, Iguchi T, Sanefuji K, Kayashima H, et al. Successful ABO incompatible living donor liver transplantation in a patient with high isoagglutinin titer using high-dose intravenous immunoglobulin. Transplant Proc. 2007; 39:3491–4.

9. Lee SD, Kim SH, Kong SY, Kim YK, Lee SA, Park SJ. ABO-incompatible living donor liver transplantation without graft local infusion and splenectomy. HPB (Oxford). 2014; 16:807–13.

10. Song GW, Lee SG, Hwang S, Kim KH, Ahn CS, Moon DB, et al. ABO-incompatible adult living donor liver transplantation under the desensitization protocol with rituximab. Am J Transplant. 2016; 16:157–70.

11. Raut V, Uemoto S. Management of ABO-incompatible living-donor liver transplantation: past and present trends. Surg Today. 2011; 41:317–22.

12. Lee CF, Cheng CH, Wang YC, Soong RS, Wu TH, Chou HS, et al. Adult living donor liver transplantation across ABO-incompatibility. Medicine (Baltimore). 2015; 94:e1796.

13. Goss MB, Rana A. ABO-incompatible liver transplantation: is it a viable option with modern innovation? Clin Liver Dis (Hoboken). 2017; 10:124–9.

14. Egawa H, Umeshita K, Uemoto S. Optimal dosage regimen for rituximab in ABO-incompatible living donor liver transplantation. J Hepatobiliary Pancreat Sci. 2017; 24:89–94.

Fig. 1.
Desensitization protocol for ABO-incompatible living donor liver transplantation. LT, liver transplantation; RIT, rituximab; PP, plasmapheresis; PGE1, prostaglandin E1; MMF, mycophenolate mofetil; IVIG, intravenous immunoglobulin; BS, basiliximab; IA, isoagglutinin.
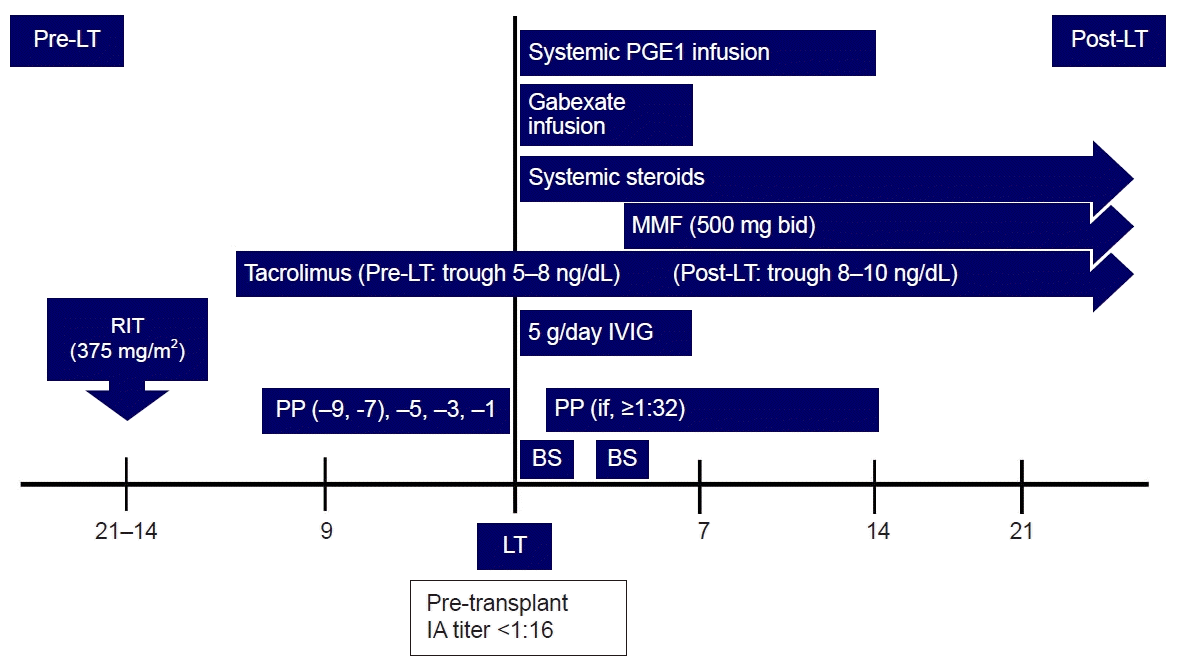
Fig. 2.
Desensitization management and IA titers in high-IA-titer ABO-incompatible living donor liver transplantation. LT, liver transplantation; IA, isoagglutinin; RIT, rituximab; PP, plasmapheresis; PGE1, prostaglandin E1; MMF, mycophenolate mofetil; IVIG, intravenous immunoglobulin; BS, basiliximab.
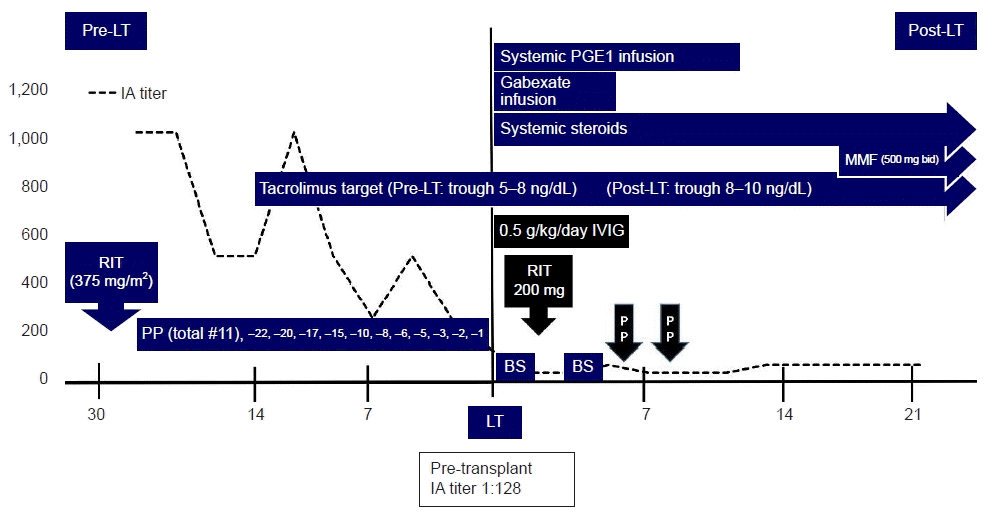
Table 1.
Changes in WBC and lymphocyte counts and CD19+ B cell and CD20+ B cell distribution according to rituximab administration




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download