Abstract
Keyhole surgery recently evolved as a minimal invasive surgical approach for treatment of anterior circulation aneurysm. This review was done to evaluate the keyhole approach for anterior circulation aneurysms, their indications, advantages, technical limitations, complications and their avoidance. The literature review was performed with the phrase “keyhole approach for anterior circulation aneurysm” as a search term in PubMed central, Medline, Google scholar and Embase data base to identify all the articles published till December 2020. Out of 113 articles searched, 22 were included in this review after screening for eligibility. On analyzing these articles, there was total 2058 aneurysm in 1871 patients. Out of 2058 aneurysm, 988 were ruptured and 547 unruptured. In 5 studies, which include 344 aneurysms in 344 cases, aneurysm ruptured or unruptured status was not specified. The most frequent aneurysm site was anterior communicating artery (n=573). The size of the aneurysm mentioned in most of the study was <15 mm. The rate of complete occlusion was ranged from 93.6-100%. The range of intra operative rupture (IOR) was 0-28.6%. The mean operative time was ranged from 70 min-5.34 hours as reported in 13 studies. Good outcome [Glasgow outcome scale (GOS): 4-5] were seen in 75-100% cases. The frontalis muscle weakness has been reported in 3 studies and ranged from 0-1.99%. Keyhole surgery can be a safe and effective treatment modality for treatment of a selected anterior circulation aneurysm. In the experienced hand it has certain advantages over standard pterional craniotomy.
The pterional craniotomy approach is the most commonly used cranial approaches, but it has certain limitations such as temporal muscle atrophy and damage to a frontal branch of facial nerve. The supraorbital keyhole approach is an alternative approach in the management of anterior skull base lesions and anterior circulation aneurysms, due to its minimal invasive nature and relatively low complication rates [31,47]. Due to advancement in the neuroradiology, microsurgical instrument and surgical techniques, the popularity of supraorbital keyhole approach for anterior circulation aneurysm is increasing.
The supra orbital keyhole approach cannot be applied for every anterior circulation aneurysm. The controversies exist regarding the use of this approach in different aneurysm location, optimal aneurysm size. Also, it is difficult to approach ruptured aneurysm due to brain swelling [11,15]. Furthermore, if an unexpected ruptures occurs intra operatively, prior to the proximal control of feeding artery, it may be very difficult for the surgeon to properly manage the bleeding. So it becomes very important for selecting suitable case for this approach.
Most of the previous studies reports on the clinical experience of keyhole technique at a single centre, which limit the generalisability of the results. In this review, the current concept and proper patient selection, advantages and limitations for this approach have been discussed to summarize the reported experience on the keyhole approach specifically in anterior circulation aneurysm.
The review was designed in accordance to the Preferred Reporting Items for Systematic Reviews and Meta-analysis.
Articles published in PubMed central, Medline, Google scholar and Embase data bases till December 2020, were all searched. In relevant literature, references were manually searched for additional articles. We screened the title and abstract by combining the term “keyhole [All Fields] AND approach [All Fields] AND anterior [All Fields] AND (“blood circulation” [MeSH Terms] OR (“blood” [All Fields] AND “circulation” [All Fields]) OR “blood circulation” [All Fields] OR “circulation” [All Fields]) AND (“aneurysm” [MeSH Terms] OR “aneurysm” [All Fields]).
Only non experimental, non animal clinical studies were included. Articles written only in English language were considered. We have included only those published articles on keyhole approach which uses supraorbital, lateral supra orbital, supercilliary, eyebrow lateral and pterional keyhole approach for treatment of anterior circulation ruptured or unruptured aneurysm. We have excluded those articles in which anterior circulation aneurysm cases were managed either by standard pterional craniotomy or by endovascular treatment. The article mentioning a modified keyhole approach, posterior circulation aneurysm, and an intracranial tumor was also excluded from the review.
Results of literature search were imported to EndNote X9 (Clarivate Analytics, PA, USA). Software utilization sought to reduce data entry errors and bias (i.e., duplicating references). All investigation reports were reviewed to assess for in consistencies (e.g., design description, outcome presentation, total patients analyzed).
Data work entered in Microsoft office excel 2007 and analyzed using SPSS version 24.0 (IBM Corp., IL, USA). Data were analyzed at two levels, descriptive and analytical. Frequency, percentage, range, means and median were used to describe the characteristics of study participants. P value <0.05 was considered statistically significant.
113 articles were identified on searching the PubMed central, Medline, Google scholer and Embase data base. Out of 113, 62 articles were screened based on removal of duplicates. After screening for eligibility of potential articles, 22 studies were included in this review review (Fig. 1).
A total 22 articles including 2058 aneurysm in 1871 patients which were surgically treated for anterior circulation aneurysm via keyhole approach met our inclusion criteria and were included in our review. The age range of these patients was 5-82 years and male to female ratio 1.42:1. The types of keyhole approaches used in these studies were supraorbital keyhole approach (SOKA), lateral supraorbital approach, supercilliary keyhole approach and pterional keyhole approach (Table 1).
Out of 2058 aneurysm, 988 were ruptured and 547 unruptured. In 5 studies, which include 344 aneurysms in 344 cases, aneurysm ruptured or unruptured status was not specified. The most frequent aneurysm sites treated in these studies were anterior communicating artery (n=573), middle cerebral artery (n=552) and posterior communicating artery (n=358). The size of the aneurysm was described differently in different study as shown in Table 2.
Out of 22 studies, 8 studies had mentioned the rate of complete occlusion which ranged from 93.6-100% (Table 3). The rate of intra operative rupture (IOR) of an aneurysm was mentioned in 11 studies, mostly either during perianeurysmal dissection or during clip application. The range of IOR in these studies was 0-28.6% cases. Yu LB et al. [51] (28.6%) and Chen L et al. [7] (26.1%) have reported maximum number of IOR. Toyooka T et al. [46] has mentioned no IOR during keyhole surgery for anterior circulation aneurysm.
There were long variation in the mean operative time (ranged: 70 min-5.34 hours) as reported in 13 studies. The mean hospital stay was reported in 7 studies (Table 3).
Glasgow outcome scale (GOS) was measured in 6 studies, ranged from 75-100%. Chalouhi N et al. [4] reported good outcome (grade 4-5) in 75% cases and Paladino J et al. [32] in 100% cases at the time of discharge. Tang Y et al. [43] reported modified Rankin Scale (mRS) of 0-2 in 71.1% of cases at 6 months of follow up. Mori K et al. [29] reported good outcome (mRS: 0-1, all patients) at 3 months of follow period.
The pterional craniotomy is most widely used surgical approach to clip the anterior circulation aneurysm [47,50]. However it requires a large scalp incision and temporalis muscle dissection which can cause facial nerve injury, depression of temporal fossa and temporo mandibular joint dysfunction. Creation of a large bone flap may result in poor cosmetic outcomes, such as keyhole site depression and the hollows of multiple burr hole sites [21,27].
The deep seated lesion in the brain can be treated by small craniotomy because it form “reverse tunnel shaped surgical corridor” with a wide operative field [28]. The evolution in the endovascular procedure with a lower complication rate and satisfactory aneurysm treatment, further inspire the surgeon to develop less morbid surgical options for aneurysm surgery. As such, less invasive surgical approachs have been proposed to reduce the extent of pterional craniotomy and expectantly its related complications [17,27]. Multiple keyhole approaches have been proposed (i.e. supraorbital and lateral supraorbital craniotomy).
The main goal of supra orbital keyhole approach is the minimization of brain exposure to air and accidental surgical trauma via limited and more specific craniotomy. Moreover, brain retraction is minimized or absent in this procedure. This significantly decreases the approach related surgical morbidity and hospitalization.
Sir Victor Horsley, first treat the cerebral aneurysm by ligation of ipsilateral common carotid artery in 1855. Dott NM started wrapping of cerebral aneurysm in 1933 [14]. Walter Dandy WE used V shaped silver clip to treat cerebral aneurysm in 1935 [13]. Yasargil first used microscope to treat intracranial aneurysm in 1960 [23]. Wilson and colleagues first operated intracranial aneurysm through a minimal craniotomy [49]. In 1978, Brock M used frontotemporal craniotomy for anterior circulation aneurysm clipping [2]. In 1982, Jane JA described the supra orbital craniotomy by opening the anterior orbital roof [19]. Later on Paladino and colleagues introduced the concept of keyhole approach [32]. In 1998, van Lindert E and colleagues introduced the supra orbital keyhole approach [47]. Various keyhole surgeries described in the literature for anterior circulation aneurysm are (i) supra orbital keyhole approach (ii) lateral supra orbital keyhole approach (iii) pterional keyhole approach (iv) superciliary keyhole approach.
Selecting the appropriate aneurysm for supra orbital keyhole approach is critical due to its technical limitations. These are as follow (Table 4) [35].
Supraorbital keyhole approach can be applied in an aneurysm with diameter up to 15 mm arising at the level of circle of willis [36]. Aneurysm arising at the posterolateral wall of the internal carotid artery (ICA), posterior communicating artery and anterior choroidal artery aneurysm, superior wall of the ICA and ICA bifurcation aneurysm can be clipped safely using supra orbital keyhole approach. Posterior wall of ICA aneurysm may necessitate placement of angled fenestrated clip encircling the ICA. It could be done with increasing the size of craniotomy. In addition, low lying supraclinoid or paraclinoid ICA aneurysm that involves drilling of anterior clinoid process also require larger craniotomies [34]. Middle cerebral artery (MCA) aneurysm arising beyond the genu requires lateral extension of the craniotomy to expose them [46]. Pterional trans-sylvian approach may require for superior directing and high positioned anterior communicating artery (AcomA) aneurysm. Posterior directing AcomA aneurysm may require anterior inter-hemispheric approach [34].
Bhattarai R et al. [1] reported the experience of 85 ruptured AcomA aneurysm via SOKA and stated that SOKA is a safe procedure in an experienced hand for the clipping of ruptured AcomA aneurysm. It provides sufficient intra operative exposure and a high clipping rate. Shin D et al. [40] treated 71 unruptured supraclinoid ICA aneurysm with diameter ranged from 4-14 mm (mean± SD: 6.6±2.3 mm) via supercilliary keyhole approach and stated that, this approach provides sufficient surgical corridor to clip most unruptured supraclinoid ICA aneurysm with advantage of short operative time, small operative wound and no post operative extra dural hematoma. Park JS et al. [37] and Chen L et al. [7] treated 188 and 88 ruptured anterior circulation aneurysm via SOKA respectively and reported that this approach has high clipping rate with good functional outcomes. Mori K et al. [29] clipped 260 unruptured anterior circulation aneurysm (63 AcomA, 150 MCA and 47 ICA aneurysms). They emphasized to use SOKA for unruptured aneurysm cases. In ruptured aneurysm cases brain swelling and sub arachnoid hemorrhage clot hinders the operating view and manipulation, so it could be avoided. Wang H et al. [48] reported the experience of 37 ruptured anterior circulation aneurysm and emphasize the use of pre operative external ventricular drainage (EVD) in ruptured anterior circulation aneurysm cases.
A limited skin incision with in the eye brow, minimal temporalis muscle dissection, small bone flap and closure with the orbicularis oculi muscle/pericranium layer have contributed to better cosmetic outcomes. Temporalis muscle atrophy, so common with standard fronto temporal and pterional craniotomy, can be avoided with eye brow incision [41]. Mori K et al. [29] reported frontalis muscle weakness in 3 (1.99%) cases. Park JS et al. [37] and Tang Y et al. [43] reported no frontalis muscle weakness out of 188 and 356 cases respectively.
Open surgical procedure for the treatment of anterior circulation aneurysm has high occlusion rate as compare to endovascular treatment [44]. Pierot L et al. [38] reported that endovascular treatment for unruptured aneurysm has complete occlusion rate 59% with 21.7% neck remnant and 19.3% aneurysm remnant. In general, aneurysm recanalization occurs more frequently with endovascular treatment than clipping. On analyzing the literature, good complete occlusion rate of aneurysm has reported with keyhole approach. Bhattarai R et al. [1] and Wang H et al. [48] reported 100% occlusion in treatment of ruptured AcomA aneurysn via SOKA. Tang Y et al. [43], Yu LB et al. [51] and Chen L et al. [7] reported 93.6%, 97.87% and 92% occlusion rate respectively for the treatment of ruptured anterior circulation aneurysm via SOKA. Toyooka T et al. [45] found 100% occlusion rate for unruptured ICA aneurysm via SOKA.
Tang Y et al. [43] and Bhattarai R et al. [1] reported mean operative time 160±57 min and 268.2±82.8 min in a study of 356 and 85 ruptured anterior circulation aneurysm respectively via SOKA. Ganesan P et al. [16] has compare supra orbital keyhole approach (41 patients) and pterional approach (82 patients) on treatment of anterior circulation aneurysm and reported that there were a significant difference in mean operative time between SOKA (192 min) and pterional approach (226 min) (p=0.007). Park JS et al. [37] also reported similar results. The less operative time in SOKA is because of smaller skin incision, smaller craniotomy and less brain exposure. Kim Y et al. [22] mentioned longer operative time (5.34±1.11 hour) as compare to other studies, this may be due to learning curve.
Tang Y et al. [43] reported average blood loss of 204±100 ml in microsurgical clipping of 356 anterior aneurysm cases by keyhole approach. Shin D et al. [40] has compare the supercilliary keyhole approach and pterional approach in the microsurgical clipping of unruptured supraclinoid ICA aneurysm and found statistically significant advantage to the supercilliary approach in terms of shorter operative time and no intra operative blood trans fusion requirement (p=0.0001). Le Roux PD et al. [24] have reported 13.2% incidence of blood transfusions during conventional pterional surgery for unruptured aneurysm. Although, most of the studies have reported that, there is less blood loss and requirement of less blood transfusion during keyhole approach for anterior circulation aneurysm but some differs. Ganesan P et al. [16] have reported statistically no significant difference in the amount of blood loss between SOKA (433 ml) and pterional approach (437 ml) (p=0.972) in the treatment of anterior circulation aneurysm. This was because there is not much difference in blood loss from the skin incision, craniotomy and dural opening. Although, the skin incision and craniotomy size are larger in the pterional approach as compare to SOKA, the most blood loss usually occurs during rupture of an aneurysm.
As compare to conventional craniotomy (7.4 days), patients treated with keyhole approach were early discharge from the hospital and matches to that of endovascular surgery (4.5 days) [18]. Caplan JM et al. [3] reported average hospital stay of 3.96 days in a study of 72 patients operated through supra orbital keyhole approach for anterior circulation aneurysm. Ganesan P et al. [16] stated that the length of surgical procedure and hospitalization were significantly reduced (p=0.007) in keyhole surgery for anterior circulation aneurysm as compare to standard technique. Similar findings were also reported by [30].
Most of the previous studies reported improved functional outcomes after keyhole surgery. Tang Y et al. [43] reported mRS of 0-2 in 71.4% cases at 6 months of follow up. Chen L et al. [7] reported GOS of 5-4 in 88.6% cases at 1 year of follow up. Mori K et al. [29] mentioned that improved outcomes may be due to absence of surgical contusion and avoidance of ischemia and hemorrhagic complications in keyhole approach.
Multiple studies have reported their experience of keyhole surgery for ruptured anterior circulation aneurysm [1,7,8,22,33,37,43,48,51]. Bhattarai R et al. [1] reported their experience of keyhole surgery of 85 ruptured AcomA aneurysm of size ranged 2-11 mm. They stated that, keyhole surgery is suitable for patients with Hunt and Hess grade I-III, including conscious patients and patients without any severe intracranial pressure. It is generally not suitable for patients with poor Hunt and Hess grade (grade IV-V) with severe complications including intracranial hypertension, brain herniation and requirement for enlargement of the bone window for decompression.
In the keyhole approach, sufficient space may be obtained for clipping ruptured aneurysm by completely removing the anterior skull base and releasing cerebrospinal fluid (CSF) from the cistern. In addition, if necessary, pre operative EVD and/or resection of part of the gyrus rectus may be performed intra operatively [11,15].
Yu LB et al. [51] performed a randomized controlled trial on keyhole craniotomy (70 cases) and pterional craniotomy (70 cases) for ruptured AcomA aneurysm. They reported that, there was lower incidence of surgical complication in keyhole group (10.5%) as compare to pterional group (32.9%). Ganesan P et al. [16] retrospectively have done a comparative study between supraorbital keyhole and pterional approach for anterior circulation aneurysm. They stated that regardless of treatment option, functional outcomes after subarachnoid haemorrhage (SAH) are dependent upon clinical presentation prior to intervention. Patients in good world federation of neurosurgical societies (WFNS) grade had good functional outcomes as compare to patients in poor WFNS score.
Small cranial opening with bimanual hand movements produces reduce light at the operating field. Angled endoscope can be used for better illumination and to look around the corners or behind structures [39].
The small size of craniotomy of 2-2.5 cm creates difficulty in maneuverability of the instruments. Single shaft, slender type of instrument including micro forceps, micro scissors and aneurysm clip appliers along with long bipolar coagulation forceps can be used to overcome this issue [35].
Small working space may create confusion in identification of neurovascular structures intra operatively. The neuro navigation tool can be used to overcome this issue [6].
The surgical corridor of dissection cannot be changed during surgery. The pre opening information regarding exact location and size of craniotomy to determine precise trajectory is an essential part of keyhole approach. The Tailor made keyhole surgery with computer simulation using 3D computed tomography angiography is useful. The various shape and sizes of virtual small craniotomy can be generated by modern work station to visualize the target lesion through the keyhole [23].
Staying at least 5 mm lateral to the supra orbital notch or foramen with the craniotomy may significantly reduce the risk of supraorbital nerve palsy [9,26]. A more medial craniotomy can be performed without damage to supraorbital nerve by dissecting below the calvarium and elevating the pericranium with the supra orbital nerve [25].
The frontalis branch of facial nerve can be injured if the incision extends greater than 13 mm lateral to the zygomatic process of frontal bone [25]. Therefore limiting the lateral extension of the incision as well as use of cautery in the temporalis muscle below the zygomatic process reduces the risk of this nerve injury [25,26].
A large frontal sinus can be injured during making supraorbital keyhole craniotomy, and may led to CSF leakage post operatively as sinus repair with pericranial patch is not possible in such a small craniotomy. So a large frontal sinus coming in planned craniotomy site of supraorbital keyhole surgery is a contraindication for keyhole surgery [23].
Keyhole surgery evolved as a minimally invasive, safe and effective treatment modality for treatment of selected ICA, AcomA and MCA aneurysms. The approach may be most suitable for properly oriented, small; preferably unruptured aneurysms of anterior circulation. In the experienced hand, keyhole approach can be used in patients with ruptured anterior circulation aneurysm in good WFNS grade. This technique has the advantages over standard pterional craniotomy such as minimal soft tissue damage with better cosmetic outcomes, satisfactory aneurysm occlusion rate, less operative time, less blood loss and patients can be discharge early from the hospital. In future, further modifications in the surgical instrument, computer simulation and advance microscope may further improve the safety of procedure.
REFERENCES
1. Bhattarai R, Liang C, Chen C, Wang H, Huang T, Ning X, et al. Supraorbital eyebrow keyhole approach for microsurgical management of ruptured anterior circulation artery aneurysm. Exp Ther Med. 2020; Sep. 20(3):2079–89.
2. Brock M, Dietz H. The small frontolateral approach for the microsurgical treatment of intracranial aneurysms. Neurochirurgia (Stuttg). 1978; Nov. 21(6):185–91.

3. Caplan JM, Papadimitriou K, Yang W, Colby GP, Coon AL, Olivi A, et al. The minipterional craniotomy for anterior circulation aneurysms: Initial experience with 72 patients. Neurosurgery. 2014; Jun. 10(Suppl 2):200–6.
4. Chalouhi N, Thakkar V, Tjoumakaris S, Fernando Gonzalez L, Hasan D, Rosenwasser R, et al. Microsurgical clipping of large and giant cerebral aneurysms: A single-center contemporary experience. J Clin Neurosci. 2014; Aug. 21(8):1424–7.

5. Cha KC, Hong SC, Kim JS. Comparison between lateral supraorbital and pterional approach in the surgical treatment of unruptured intracranial aneurysms. J Korean Neurosurg Soc. 2012; Jun. 51(6):334–7.

6. Chalouhi N, Jabbour P, Ibrahim I, Starke RM, Younes P, El Hage G, et al. Surgical treatment of ruptured anterior circulation aneurysms: Comparison of pterional and supraorbital keyhole approaches. Neurosurgery. 2013; Mar. 72(3):437–41.
7. Chen L, Tian X, Zhang J, Huang Y, Chen E, Lan Q. Is eyebrow approach suitable for ruptured anterior circulation aneurysms on early stage: A prospective study at a single institute? Acta Neurochir (Wien). 2009; Jul. 151(7):781–4.

8. Chen Z, Sun X, Lu T, Lu Z, Jiang M, Zhao C, et al. Comparison between modified lateral supraorbital approach and pterional approach in the surgical treatment of middle cerebral artery aneurysms. Chin Neurosurg J. 2018; Feb. 4:4.

9. Cheng C, Noguchi A, Dogan A, Anderson GJ, Hsu FPK, McMenomey SO, et al. Quantitative verification of the keyhole concept: A comparison of area of exposure in the parasellar region via supraorbital keyhole, frontotemporal pterional, and supraorbital approaches. J Neurosurg. 2013; Feb. 118(2):264–9.

10. Cheng W-Y, Lee H-T, Sun M-H. A pterion keyhole approach for the treatment of anterior circulation aneurysms. Minim Invasive Neurosurg. 2006; Oct. 49(5):257–62.

11. Choi JH, Kang MJ, Huh JT. Influence of clinical and anatomic features on treatment decisions for anterior communicating artery aneurysms. J Korean Neurosurg Soc. 2011; Aug. 50(2):81–8.

12. Choi YJ, Son W, Park KS, Park J. Intradural procedural time to assess technical difficulty of superciliary keyhole and pterional approaches for unruptured middle cerebral artery aneurysms. J Korean Neurosurg Soc. 2016; Nov. 59(6):564–9.

13. Dandy WE. Intracranial aneurysm of the internal carotid artery: Cured by operation. Ann Surg. 1938; May. 107(5):654–9.

14. Dott NM. Intracranial aneurysms: Cerebral arterio-radiography: Surgical treatment. Edinb Med J. 1933; Dec. 40(12):T219–40.
15. Froelich S, Cebula H, Debry C, Boyer P. Anterior communicating artery aneurysm clipped via an endonasal approach: Technical note. Neurosurgery. 2011; Jun. 68(2 Suppl Operative):310–6.
16. Genesan P, Haspani MSM, Noor SRM. A comparative study between supraorbital keyhole and pterional approach on anterior circulation aneyrysms. Malays J Med Sci. 2018; Sep. 25(5):59–67.

17. Harland SP, Hussein A, Gullan RW. Modification of the standard pterional approach for aneurysms of the anterior circle of Willis. Br J Neurosurg. 1996; Apr. 10(2):149–53.

18. Higashida RT, Lahue BJ, Torbey MT, Hopkins LN, Leip E, Hanley DF. Treatment of unruptured intracranial aneurysms: A nationwide assessment of effectiveness. AJNR Am J Neuroradiol. 2007; Jan. 28(1):146–51.
19. Jane JA, Park TS, Pobereskin LH, Winn HR, Butler AB. The supraorbital approach: Technical note. Neurosurgery. 1982; Oct. 11(4):537–42.

20. Kang HJ, Lee YS, Suh SJ, Lee JH, Ryu KY, Kang DG. Comparative analysis of the mini pterional and supraorbital craniotomies for unruptured aneurysms with numeric measurements of their geometric configurations. J Cerebrovasc Endovasc Neurosurg. 2013; Mar. 15(1):5–12.

21. Kil JS, Kim DW, Kang SD. Navigation-guided keyhole approach for unruptured intracranial aneurysms. Korean J Cerebrovasc Surg. 2011; Sep. 13(3):244–8.
22. Kim Y, Yoo CJ, Park CW, Kim MJ, Choi DH, Kim YJ, et al. Modified supraorbital keyhole approach to anterior circulation aneurysms. J Cerebrovasc Endovasc Neurosurg. 2016; Mar. 18(1):5–11.

23. Krayenbühl HA, Yaşargil MG, Flamm ES, Tew JM Jr. Microsurgical treatment of intracranial saccular aneurysms. J Neurosurg. 1972; Dec. 37(6):678–86.

24. Le Roux PD, Elliott JP, Winn HR. Blood transfusion during aneurysm surgery. Neurosurgery. 2001; Nov. 49(5):1068–74.

25. Lin Y, Zhang W, Luo Q, Jiang J, Qiu Y. Extracranial microanatomic study of supraorbital keyhole approach. J Craniofac Surg. 2009; Jan. 20(1):215–8.

26. Mitchell P, Vindlacheruvu RR, Mahmood K, Ashpole RD, Grivas A, Mendelow AD. Supraorbital eyebrow minicraniotomy for anterior circulation aneurysms. Surg Neurol. 2005; Jan. 63(1):47–51.

27. Miyazawa T. Less invasive reconstruction of the temporalis muscle for pterional craniotomy: Modified procedures. Surg Neurol. 1998; Oct. 50(4):347–51.

28. Mori K. Keyhole concept in cerebral aneurysm clipping and tumor removal by the supraciliary lateral supraorbital approach. Asian J Neurosurg. 2014; Jan. 9(1):14–20.

29. Mori K, Wada K, Otani N, Tomiyama A, Toyooka T, Fujii K, et al. Validation of effectiveness of keyhole clipping in nonfrail elderly patients with unruptured intracranial aneurysms. J Neurosurg. 2017; Dec. 127(6):1307–14.

30. Nathal E, Gomez-Amador JL. Anatomic and surgical basis of the sphenoid ridge keyhole approach for cerebral aneurysms. Neurosurgery. 2005; Jan. 56(1 Suppl):178–85.

31. Paladino J, Mrak G, Miklić P, Jednacak H, Mihaljević D. The keyhole concept in aneurysm surgery—a comparative study: Keyhole versus standard craniotomy. Minim Invasive Neurosurg. 2005; Oct. 48(5):251–8.

32. Paladino J, Pirker N, Stimac D, Stern-Padovan R. Eyebrow keyhole approach in vascular neurosurgery. Minim Invasive Neurosurg. 1998; Dec. 41(4):200–3.

33. Park HS, Park SK, Han YM. Microsurgical experience with supraorbital keyhole operations on anterior circulation aneurysms. J Korean Neurosurg Soc. 2019; Aug. 46(2):103–8.

34. Park J. Superciliary keyhole approach for unruptured anterior circulation aneurysms: Surgical technique, indications, and contraindications. J Korean Neurosurg Soc. 2014; Nov. 56(5):371–4.

35. Park J. Supraorbital keyhole approach for intracranial aneurysms: Transitioning from concern to confidence. J Korean Neurosurg Soc. 2020; Jan. 63(1):4–13.

36. Park J, Woo H, Kang DH, Sung JK, Kim Y. Superciliary keyhole approach for small unruptured aneurysms in anterior cerebral circulation. Neurosurgery. 2011; Jun. 68(2 Suppl Operative):300–9.

37. Park JS, Kim H, Baik MW, Park IS. Risk factor analysis for poor outcomes in supraorbital keyhole aneurysm clipping for ruptured anterior circulation aneurysms. World Neurosurg. 2018; Mar. 111:e386–94.

38. Pierot L, Spelle L, Vitry F; ATENA Investigators. Immediate clinical outcome of patients harboring unruptured intracranial aneurysms treated by endovascular approach: Results of the ATENA study. Stroke. 2008; Sep. 39(9):2497–504.

39. Reisch R, Perneczky A. Ten-year experience with the supraorbital subfrontal approach through an eyebrow skin incision. Neurosurgery. 2005; Oct. 57(suppl):242–55.

40. Shin D, Park J. Unruptured supraclinoid internal carotid artery aneurysm surgery: Superciliary keyhole approach versus pterional approach. J Korean Neurosurg Soc. 2012; Oct. 52(4):306–11.

41. Steiger H-J, Schmid-Elsaesser R, Stummer W, Uhl E. Transorbital keyhole approach to anterior communicating artery aneurysms. Neurosurgery. 2001; Feb. 48(2):347–52.

42. Tang C, Sun J, Xue H, Yu Y, Xu F. Supraorbital keyhole approach for anterior circulation aneurysms. Turk Neurosurg. 2013; 23(4):434–8.
43. Tang Y, Chen H, Ma F, Scharnweber R, Zhang J, Chen G, et al. Experience using the pterional keyhole approach for the treatment of ruptured intracranial aneurysms of the anterior circulation. World Neurosurg. 2018; Oct. 118:e800–5.

44. Thompson BG, Brown RD Jr, Amin-Hanjani S, Broderick JP, Cockroft KM, Connolly ES Jr, et al. Guidelines for the management of patients with unruptured intracranial aneurysms: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015; Aug. 46(8):2368–400.

45. Toyooka T, Wada K, Otani N, Tomiyama A, Takeuchi S, Tomura S, et al. Potential risks and limited indications of the supraorbital keyhole approach for clipping internal carotid artery aneurysms. World Neurosurg X. 2019; Feb. 2:100025.

46. Tra H, Huynh T, Nguyen B. Minipterional and supraorbital keyhole craniotomies for ruptured anterior circulation aneurysms: Experience at single center. World Neurosurg. 2018; Jan. 109:36–9.

47. van Lindert E, Perneczky A, Fries G, Pierangeli E. The supraorbital keyhole approach to supratentorial aneuryms: Concept and technique. Surg Neurol. 1998; May. 49(5):481–9.
48. Wang H, Chen C, Ye ZP, Luo L, Li W-S, Guo Y. On clipping of anterior communicating artery aneurysms via eyebrow lateral keyhole approach. Int J Clin Exp Med. 2015; Nov. 8(11):21114–21.
49. Wilson DH. Limited exposure in cerebral surgery. Technical note. J Neurosurg. 1971; Jan. 34(1):102–6.
Fig. 1.
Flow chart (preferred reporting items for systematic review and meta-analysis) for articles selection (original figures).
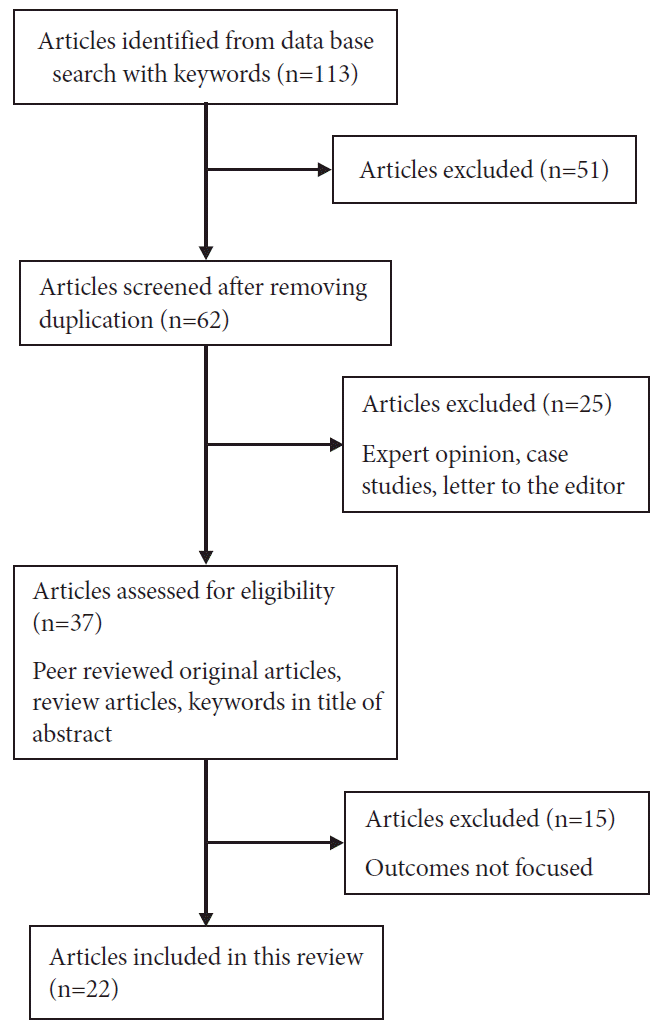
Table 1.
Previous studies and patients characteristics
| S. no. | Studies | No of patients/no of aneurysms | Keyhole approach | Age in years | Male/female |
|---|---|---|---|---|---|
| 1 | Bhattarai R et al., 2020. [1] | 85/85 | SOK | 28-78 | 50/35 |
| Mean±SD: 52.69±9.94 | |||||
| 2 | Toyooka T et al., 2019. [45] | 51/51 | SOK | 35-75 | 24/27 |
| Mean 62.0 | |||||
| 3 | Tang Y et al., 2018. [43] | 356/408 | PKA | NA | NA |
| 4 | Genesan P et al., 2018. [16] | 41/41 | SOK | 26-82 | 19/22 |
| 54.95±12.71 | |||||
| 5 | Park JS et al., 2018. [37] | 188/188 | SKA | NA | NA |
| 6 | Yu LB et al., 2015. [51] | 47/47 | PKA | NA | NA |
| 7 | Chin Z et al., 2018. [8] | 27/27 | Lateral supraorbital approach | 39-73 | 21/6 |
| 56.1±10.1 | |||||
| 8 | Mori K et al., 2017. [29] | 151/260 | PKA+SOK | NA | NA |
| 9 | Kim Y et al., 2016. [22] | 40/40 | SOK | 57.10±11.77 | 20/20 |
| 10 | Choi YJ et al., 2016. [12] | 124/124 | Superciliary keyhole approach | 36-74 | 81/43 |
| 50.4±9.2 | |||||
| 11 | Wang H et al., 2015. [48] | 37/37 | Eyebrow lateral keyhole | 5-76 | 24/13 |
| 46.3 | |||||
| 12 | Caplan JM et al., 2014. [3] | 72/82 | PKA | NA | NA |
| 13 | Chalouhi N et al., 2013. [4] | 87/87 | SKA+PKA | <12 mm 80% | NA |
| 14 | Tang C et al., 2013. [42] | 76/80 | SKA | NA | NA |
| 15 | Kang HJ et al., 2013. [20] | 4/4 | SOK | Mean 58.5 | NA |
| 16 | Shin D et al., 2012. [40] | 70/71 | Superciliary keyhole approach | 33-65 | NA |
| 57.0 | |||||
| 17 | Cha KC et al., 2012. [5] | 61/68 | Lateral supraorbital | Mean 54.6 | 33/28 |
| 18 | Chen L et al., 2009. [7] | 88/88 | SKA | NA | NA |
| 19 | Park HS et al., 2009. [33] | 50/54 | NA | 55.7±12.3 | 31/19 |
| 20 | Cheng WY et al., 2006. [10] | 40/40 | PKA | NA | NA |
| 21 | Paladino J et al., 1998. [32] | 37/37 | SOEK | NA | NA |
| 22 | van Lindert E et al., 1998. [47] | 139/139 | SOEK | NA | NA |
| Total | 1871/2058 |
Table 2.
Aneurysm characteristics
| S. no. | Studies | R/U | AcomA | PcomA | AChA | ICA bif | ICA Paraclinoid | MCA | Size (mm) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Bhattarai R et al., 2020. [1] | 85/0 | 85 | 0 | 0 | 0 | 0 | 0 | 2-11 |
| 5.07±2.36 | |||||||||
| 2 | Toyooka T et al., 2019. [45] | 0/51 | 0 | 32 | 15 | 2 | 2 | 0 | 2-10 |
| 5.8±1.8 | |||||||||
| 3 | Tang Y et al., 2018. [43] | 356/0 | 118 (ACA 18) | 105 | 9 | NA | 26 | 50 | <25 mm |
| 4 | Genesan P et al., 2018. [16] | NA | NA | NA | NA | NA | NA | NA | NA |
| 5 | Park JS et al., 2018. [37] | 188/0 | 82 | 44 | 2 | 3 | 1 | 51 | Avg 5.5 mm |
| 6 | Yu LB et al., 2015. [51] | 47/0 | 10, ACA-3 | 15 | 0 | 0 | 0 | 19 | <20 mm |
| 7 | Chin Z et al., 2018. [8] | 27/0 | 0 | 0 | 0 | 0 | 0 | 27 | NA |
| 8 | Mori K et al., 2017. [29] | 0/151 | 63 | 0 | 0 | 0 | 47 | 150 | <10 |
| 9 | Kim Y et al., 2016. [22] | 40/0 | NA | NA | NA | NA | NA | NA | NA |
| 10 | Choi YJ et al., 2016. [12] | 0/124 | 0 | 0 | 0 | 0 | 0 | 124 | 3-14.6 |
| 5.1±2.1 | |||||||||
| 11 | Wang H et al., 2015. [48] | 37/0 | 37 | 0 | 0 | 0 | 0 | 0 | 1.4-16 |
| 4.8 | |||||||||
| 12 | Caplan JM et al., 2014. [3] | 0/72 | 0 | 22 | 1 | 0 | 23 | 36 | Avg 5.45 |
| 13 | Chalouhi N et al., 2013. [4] | NA | 51 | 26 | 0 | 0 | 7 | 0 | NA |
| 14 | Tang C et al., 2013. [42] | 70/6 | 33, A1- 4 | 14 | 0 | 0 | 5 | 17 | 5-19 mm |
| 15 | Kang HJ et al., 2013. [20] | 0/4 | 2 | 2 | 0 | 0 | 0 | 0 | NA |
| 16 | Shin D et al., 2012. [40] | 0/71 | 0 | 45 | 17 | 0 | 4 | 0 | 4-14 |
| 6.6±2.3 | |||||||||
| 17 | Cha KC et al., 2012. [5] | 0/68 | 16+3 (A1 seg) | 8 | 8 | 3 | 1 (oph seg) | 29 | NA |
| 18 | Chen L et al., 2009. [7] | 88/0 | 41 | 18 | 0 | 0 | 0 | 29 | <15 mm |
| 100% | |||||||||
| 19 | Park HS et al., 2009. [33] | 50/0 | 26 | 13 | 1 | 2 | 2 (oph seg) | 10 | NA |
| 20 | Cheng WY et al., 2006. [10] | NA | 9+1 (ACA) | 14 | 0 | 0 | 6 | 10 | NA |
| 21 | Paladino J et al., 1998. [32] | NA | NA | NA | NA | NA | NA | NA | NA |
| 22 | van Lindert E et al., 1998. [47] | NA | NA | NA | NA | NA | NA | NA | NA |
| Total | 988/547 | 573+29 (ACA) | 358 | 53 | 10 | 124 | 552 |
Table 3.
Intra operative findings and post operative outcomes
| S.no. | Studies | Complete occulusion | Intraoperative rupture | Operative time (min) | Hospital stay (days) | GOS | mRS | Frontalis weakness |
|---|---|---|---|---|---|---|---|---|
| 1 | Bhattarai R et al., 2020. [1] | 100% | 3.53% | 60-360 | 37.9±24.5 | NA | NA | NA |
| 268.2±82.8 | ||||||||
| 2 | Toyooka T et al., 2019. [45] | 100% | 0 | 122-273 | 3.4±6.9 | NA | NA | NA |
| 171±33 | ||||||||
| 3 | Tang Y et al., 2018. [43] | 93.60% | NA | 160±57 | 8.33±2.72 | NA | 6 months | 0 |
| 0-2,71.1% | ||||||||
| 4 | Genesan P et al., 2018. [16] | NA | NA | 192 mean | NA | NA | NA | NA |
| 5 | Park JS et al., 2018. [37] | NA | 1.06% | NA | NA | Good outcome 91.5% | NA | 0 |
| 6 | Yu LB et al., 2015. [51] | 97.87% | 28.6% | NA | 15.5 | Good outcome 83.6% | NA | NA |
| 7 | Chin Z et al., 2018. [8] | NA | NA | 146±36.7 m | NA | NA | NA | NA |
| 8 | Mori K et al., 2017. [29] | 96% | NA | 177±39 | 2.7±4.7 | NA | 0.0±0.3 | 1.99% |
| 9 | Kim Y et al., 2016. [22] | NA | NA | 5.34±1.11 hour | 18.2±7.05 | NA | NA | NA |
| 10 | Choi YJ et al., 2016. [12] | NA | NA | 7.8-59 m | NA | NA | NA | NA |
| 29.3±12.3 | ||||||||
| 11 | Wang H et al., 2015. [48] | 100% | 8.11% | 1.5-3.5 hour | NA | NA | NA | NA |
| Mean 2.5 hour | ||||||||
| 12 | Caplan JM et al., 2014. [3] | NA | NA | NA | 3.96 | NA | NA | NA |
| 13 | Chalouhi N et al., 2013. [4] | NA | 6.90% | PKA 256 m | NA | Good outcome 75% | NA | NA |
| SKA 205 m | ||||||||
| 14 | Tang C et al., 2013. [42] | 94% | 10.5% | NA | NA | Discharge 4-5; 95% | NA | NA |
| 15 | Kang HJ et al., 2013. [20] | NA | NA | NA | NA | NA | NA | NA |
| 16 | Shin D et al., 2012. [40] | NA | NA | 70-160 m | NA | NA | NA | NA |
| 99.9±17.7 | ||||||||
| 17 | Cha KC et al., 2012. [5] | NA | NA | Mean 117.1 m | NA | NA | NA | NA |
| 18 | Chen L et al., 2009. [7] | 92% | 26.1% | NA | NA | 1 year 5-4, 88.6% | NA | NA |
| 19 | Park HS et al., 2009. [33] | NA | NA | 70-187 | NA | NA | NA | NA |
| mean 160 | ||||||||
| 20 | Cheng WY et al., 2006. [10] | NA | 7.5% | NA | NA | NA | NA | NA |
| 21 | Paladino J et al., 1998. [32] | NA | 8.1% | NA | NA | Good outcome 100% | NA | NA |
| 22 | van Lindert E et al., 1998. [47] | NA | 2.1% | NA | NA | NA | NA | None |
Table 4.
Indication and contraindication




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download