Abstract
Ruptured giant aneurysms in the posterior circulation with poor grade subarachnoid haemorrhage (SAH) are associated with poor outcome. In this report four patients with ruptured giant vertebral artery aneurysms who presented acutely with World Federation of Neurosurgical Societies (WFNS) grade five SAH are reviewed. All 4 cases required intubation and ventilation on arrival. Brainstem reflexes were intact in all of them. Early endovascular parent artery coil occlusion was done in two cases. Two other cases were treated with early surgical proximal parent artery clip occlusion. Two cases required ventriculoperitoneal shunting. All cases achieved good recovery with full functional independent outcome at two years follow up. MR angiogram at two years documented resolution of aneurysms. In conclusion good outcome may be possible in some cases of ruptured giant vertebral artery aneurysms with WFNS grade five SAH.
Giant aneurysms are aneurysms larger than 25mm. In the Unruptured Cerebral Aneurysm Study of Japan only 0.5% of intracranial aneurysms are giants and only 1.8% of aneurysms are located in the vertebral artery [8]. In the International Subarachnoid Aneurysm Trial (ISAT) study posterior circulation aneurysms accounted for only 2.7% of ruptured intracranial aneurysms with subarachnoid haemorrhage [7]. In a study reported by Barrow Neurological Institute only one of 31 aneurysms (3%) of the vertebral artery is giant in size [6]. So overall ruptured giant vertebral artery aneurysms are very uncommon. Due to poor prognosis with World Federation of Neurosurgical Societies (WFNS) grade five subarachnoid haemorrhage (SAH) and the challenging task of treatment for posterior circulation giant aneurysm, coupled with computed tomography (CT) angiogram or magnetic resonance imaging (MRI) findings of clots with severe brainstem mass effects, conservative approach may sometimes be adopted [1].
Currently the management of giant aneurysms include reconstructive or deconstructive techniques. Surgical clipping of aneurysm sac or aneurysmorrhaphy eliminates the aneurysm sac from the circulation and preserve the flow in the parent artery [4]. Endovascular reconstructive techniques include stent assisted coiling of aneurysm or flow diversion with some coil packing in the giant aneurysm [2,3]. Deconstructive techniques include surgical or endovascular proximal occlusion or trapping. In some cases, bypass procedures may be required to provide collateral flow if parent artery sacrifice could lead to hemodynamic insufficiency. There are relative merits and potential complications in the use of the various therapeutic options depending on the size, configuration, and location of the aneurysm as well as the branching and collateral flow pattern in the individual patient. The paired vertebral arteries are unique in the sense that for aneurysms affecting the non-dominant or co-dominant vertebral artery, it is safe to perform parent artery sacrifice. The ability to perform safe parent artery occlusion is also dependent on the involvement of the origin or relative locations of the origin of the posterior inferior cerebellar artery (PICA), anterior inferior cerebellar artery (AICA) and the anterior spinal artery (ASA). This study reports four cases with giant vertebral artery aneurysms presenting acutely with WFNS grade 5. The interventions administered were endovascular or surgical proximal parent artery occlusion. All the cases received early intubation as well as full hypertensive hypervolemic intensive care support for SAH.
Eighteen years old boy with no past medical history presented with sudden severe headache followed by rapidly declining conscious level. When he arrived at the hospital, he was totally unresponsive with Glasgow coma score (GCS) of 3. His pupils and brainstem reflexes were reactive. He had pink frothy pulmonary secretions and borderline oxygen saturation (signs of acute pulmonary oedema). He deteriorated rapidly and developed cardiac arrest. Cardiopulmonary resuscitation was instituted and he was intubated and ventilated. He also required inotropic support. Clinically there were no evidence of polycystic kidney disease, coarctation of aorta or connective tissue disorder. Urgent CT angiogram following hemodynamic stabilisation showed Fisher grade 4 SAH with clots in the posterior fossa and ventricles, giant left vertebral artery aneurysm with brainstem compression as well as hydrocephalus (Fig. 1). Catheter angiogram showed giant left vertebral artery aneurysm with PICA origin distal to the aneurysm (Fig. 2). The contralateral right vertebral artery was dominant. The ASA was not visualised. Early ventricular drainage followed by proximal coil occlusion of the left vertebral artery was performed two hours later. 6F Envoy guiding catheter (Johnson and Johnson, NJ, USA) was inserted in the mid left vertebral artery. SL 10 microcatheter (Stryker, MI, USA) was navigated with a Synchro 14 micro-guide wire (Stryker, MI, USA) just proximal to the aneurysm. Coil occlusion was performed with multiple coils just proximal to the aneurysm (Fig. 3). Apart from routine heparin flush (500 units in 1 L normal saline bag), no systemic anticoagulation was given in view of the ventriculostomy and the possible need for decompressive craniectomy or shunt procedures later. For the same reasons no antiplatelet medications were administered. He had a very stormy, prolonged course in the intensive care and he subsequently underwent tracheostomy and ventriculoperitoneal shunting.
Postoperative CT 3 weeks post coil occlusion of the left vertebral artery showed reduction of mass effect in the posterior fossa (Fig. 4). At 6 months’ follow up he was noted to have surprisingly good recovery. He was functionally fully independent with no neurological deficit and he returned to full time study in tertiary education at 1 year. Two year follow up MRI showed resolution of the aneurysm with no flow in the ipsilateral vertebral artery distal to the site of endovascular coil occlusion. Contralateral vertebral artery provided antegrade flow into basilar artery and the rest of the posterior circulation.
Twenty-nine years old lady with no past medical history presented with sudden severe headache and deteriorating conscious level. Upon arrival she was flexing to painful stimulus with no eye opening or verbal response. Pupillary and brainstem reflexes were preserved. Urgent CT angiogram showed Fisher grade 4 SAH with posterior fossa clot, blood in fourth and lateral ventricles but no hydrocephalus. There was a right giant vertebral artery aneurysm with severe brainstem compression (Fig. 5). There was no obvious PICA, AICA or ASA in the ipsilateral, dysplastic intracranial vertebral artery. The contralateral vertebral artery was co-dominant. Far lateral approach [3] with wide craniectomy and removal of ipsilateral half of C1 arch was performed 2 hours later. Extradural segment of vertebral artery was dissected out early for proximal control. Proximal aneurysm clip ligation with Sugita titanium aneurysm clips (Mizuho, Tokyo, Japan) was performed after exposure of the proximal aneurysm neck. No attempt was made to expose the entire aneurysm or vertebral artery distal to the aneurysm. Lumbar drain was inserted intraoperatively for cerebrospinal fluid diversion to alleviate raised intracranial pressure. She required prolonged stay in the intensive care unit over two weeks but she did not require tracheostomy. She did not require ventriculoperitoneal shunting either. At two years’ follow up she was fully independent and returned to work as a technician at one year. Magnetic resonance angiography (MRA) at two years follow up showed progressive shrinkage of the aneurysm with no flow in the ipsilateral vertebral artery distal to the site of clip occlusion (Fig. 6).
Fifty years old male with no past medical history was found to be unconscious. Upon arrival at the hospital he was flexing to painful stimulus with no eye opening or verbal response. Brainstem reflexes were preserved. He was intubated and ventilated for airway control. Urgent MRI showed a right vertebral artery giant aneurysm measuring 5 cm at its largest dimension with subarachnoid haemorrhage (Fig. 7). There was brainstem distortion but no hydrocephalus. Catheter angiogram demonstrated partial filling of the giant aneurysm. The ipsilateral PICA arose distal to the aneurysm (Fig. 8). ASA was not visualised. The contralateral vertebral artery was co-dominant. Proximal clip parent artery occlusion was performed with far lateral suboccipital approach with the same technique as described in Case 2. A lumbar drain was inserted intraoperatively for cerebrospinal fluid diversion to alleviate raised intracranial pressure. He required prolonged stay in the intensive care unit for three weeks. He did not require tracheostomy or ventriculoperitoneal shunting. Delayed cerebral angiogram at 6 weeks showed occlusion of right vertebral artery with no filling of aneurysm. The left vertebral artery angiogram showed retrograde filling of the distal right vertebral artery and right PICA with no opacification of aneurysm. There was good filling of basilar artery and the rest of the posterior circulation from the left vertebral artery (Fig. 9). At two year follow up he was fully independent and returned to work as a shop keeper. Two year follow up MRI showed resolution of aneurysm.
Twenty-seven years old male factory worker with no past medical history presented with sudden severe headache, vomiting and deteriorating conscious level. Upon arrival at the hospital he was extending to pain with no eye opening or verbal response. Brainstem reflexes were preserved. He was intubated and ventilated. Urgent CT scan showed Fisher grade 4 SAH with haematoma in the posterior fossa distorting the brainstem and intraventricular blood. There was no hydrocephalus. Cerebral angiography showed a large 35 mm giant aneurysm in the co-dominant left vertebral artery (Fig. 10). The aneurysm neck was at the level of mid clivus and the PICA origin was located proximally at the level of foramen magnum. Early endovascular coil occlusion of the left vertebral artery was performed proximal to the aneurysm in-flow zone and distal to the PICA. Postop angiogram documented no filling of the aneurysm and preservation of flow in the ipsilateral PICA (Fig. 11). Contralateral vertebral artery injection showed good flow into the basilar artery and the rest of posterior circulation with no retrograde filling of the aneurysm. He required tracheostomy and stayed in the intensive care unit for three weeks. He also required ventriculoperitoneal shunting for delayed hydrocephalus. At 6 months follow up he was fully independent. He returned to work one year post ictus. MRA at two years showed resolution of the aneurysm.
Poor grade SAH from ruptured giant aneurysms in the posterior fossa carries poor prognosis. Currently apart from cerebrospinal fluid diversion procedures to relief intracranial pressure, surgical or endovascular interventionists are hesitant to treat due to high risk of complications and poor outcome even when interventions were successful [1].
The treatment strategies for giant aneurysms today include reconstructive or deconstructive approaches, surgically or endovascularly [5].
Surgical reconstructive techniques include direct clipping of aneurysm neck or aneurysmorrhaphy. These two approaches are very challenging technically in the setting of severe brain swelling during the acute phase of acute SAH coupled with the severe mass effect of the aneurysm. High risk of haemorrhage during the dissection in between cranial nerves to achieve distal control during the acute phase of SAH made it an even harder uphill task. In fact, hypothermic circulatory arrest was advocated by some centres to allow for a safe period of bloodless field for the direct repair of these very difficult aneurysms [6].
Surgical proximal occlusion is simpler and carries less complications because there is no need for dissection beyond the proximal aneurysm neck when it is adopted as the definitive strategy. The main requirement is the presence of contralateral dominant or co-dominant vertebral artery (although bypass procedures such as superficial temporal artery to superior cerebellar artery is feasible it is usually not advocated in the acute settings). Cerebrospinal fluid diversion and decompressive craniectomy can be performed at the same time as proximal occlusion under the same general anaesthesia for surgery if indicated to improve cerebral perfusion. These are potentially even more helpful should the patient develop vasospasm subsequently. The important angiographic anatomy to pay attention to are the locations of the origin of PICA and ASA. Prior to surgery (if it is adopted as intervention of choice) it is also important to note the small percentage of extradural origin of PICA. Otherwise this important vessel may be damaged during exposure leading to lateral medullary syndrome. In Case 2 and 3, due to absence of ventriculomegaly lumbar drainage were carried out following craniectomy prior to dura opening (to avoid the risks of coning). Far lateral approach [3] with wide craniectomy and removal of ipsilateral half of C1 arch were performed. Early exposure of the extradural segment of the vertebral artery is useful to achieve early proximal control. Proximal aneurysm clip ligation with titanium aneurysm clips were performed after exposure of the proximal aneurysm neck. No attempt was made to expose the entire aneurysm or vertebral artery distal to the aneurysm. Utmost care was taken to avoid cranial nerve injury. Wide craniectomy and partial removal of the arch of C1 allows early proximal control and wider angle of choice in the placement of the clip to reduce complications. There is possibility of retrograde filling of the aneurysm leading to recanalization or rebleeding. However, endovascular occlusion of the distal neck can be performed later from contralateral vertebral artery or via patent posterior communicating artery-P1 segment of posterior cerebral artery from the top should the need arise when the patient recovers from the primary effects of SAH. The main disadvantages for surgical approach are longer duration, blood loss, intraoperative rupture from manipulation, perforator injury as well as cranial nerve injury. The advantages are the ability to decompress posterior fossa, cerebrospinal fluid diversion and no necessity for antiplatelet medications or anticoagulation.
Endovascular deconstructive techniques include proximal coil occlusion, coiling of the aneurysm sac plus proximal coil occlusion as well as trapping with coils proximal and distal to the aneurysm [3,5]. For giant aneurysms with mass effect in the posterior fossa in acute haemorrhagic setting, proximal coil occlusion is the simplest and most practical. Placement of coils in the aneurysm worsens the mass effect. And based on current understanding of aneurysm flow dynamics, with reversal of flow after proximal occlusion there is reasonable chance of aneurysm resolution with proximal occlusion alone. For these reasons proximal coil occlusion is preferred. There is also added advantage that anticoagulation or antiplatelet medications are not required.
Endovascular reconstructive techniques include stent assisted coiling or flow diversion with or without coils. Both require double antiplatelets. Sometimes for giant aneurysms of other intracranial locations where mass effect is not as critical as the posterior fossa early stage partial coiling followed by delayed stenting or flow diverter insertion after a period of antiplatelet loading have been performed [2,3]. For ruptured poor grade giant vertebral aneurysms these approaches may have limited application except when the contralateral vertebral artery is absent or very small in calibre. There are also documented recurrences despite initial successful stent assisted coiling or flow diversion. In acute haemorrhagic setting when there is a significant potential need for cerebrospinal fluid diversion procedure the dual antiplatelet therapy elevates the risks of haemorrhage when cerebrospinal fluid diversion or the need for revision when the drains are blocked are significant.
In terms of aetiology for the aneurysms, these 4 cases did not have connective tissue disease, polycystic kidney or coarctation of aorta base on clinical history and examination. There were also no definitive double lumen sign or intimal flaps as proofs of intracranial dissection.
Since the publication of ISAT study results there has been a trend favouring endovascular compared to surgical approach for ruptured posterior circulation aneurysms [7]. In Case 1 due to poor GCS, acute pulmonary oedema with haemodynamic instability, fast endovascular proximal occlusion was chosen. For Cases 2, 3 and 4 the ability to perform proximal occlusion close to the aneurysm is equal whether endovascular or surgical approach was chosen. It is speculated that surgical or endovascular proximal occlusion probably would have produced similar outcomes for the three of them.
It is also worth noting that endovascular parent artery occlusion can be performed after cerebrospinal fluid diversion procedures and / or craniectomy and cerebrospinal fluid diversion procedures. Conversely decompressive craniectomy can be performed following endovascular proximal occlusion. They are not mutually exclusive. In the same vein if surgical proximal clipping is not feasible or unsuccessful, endovascular proximal occlusion can be performed. If endovascular access is not feasible for one reason or another, surgical proximal clipping can be performed.
All 4 cases received early intensive care support and early endovascular or surgical intervention to prevent rebleeding. The youngest Case 1 returned to tertiary education after a year. The other three cases took two years to return to work. They were all 50 years old or below with no underlying medical morbidity. The ability of all of them to return to pre-morbid occupation was encouraging when the worse outcome was expected.
REFERENCES
1. Batjer H. Giant aneurysms of the posterior circle of Willis and its branches. In : Awad I, Barrow D, editors. Giant Intracranial Aneurysms. American Association of Neurological Surgeons;1995. p. 175–96.
2. Brinjikji W, Piano M, Fang S, Pero G, Kallmes DF, Quilici L, et al. Treatment of ruptured complex and large/giant ruptured cerebral aneurysms by acute coiling followed by staged flow diversion. J Neurosurg. 2016; Jul. 125(1):120–7.

3. Cagnazzo F, Mantilla D, Rouchaud A, Brinjikji W, Lefevre PH, Dargazanli C, et al. Endovascular treatment of very large and giant intracranial aneurysms: comparison between reconstructive and deconstructive techniques-a meta-analysis. AJNR Am J Neuroradiol. 2018; May. 39(5):852–8.

4. Campos JK, Ball BZ, II BC, Sweidan AJ, Hasjim BJ, Hsu FPK, et al. Multimodal management of giant cerebral aneurysms: review of literature and case presentation. Stroke and Vascular Neurology. 2020; Mar. 5(1):22–8.

5. Gmeiner M, Gruber A. Current strategies in the treatment of intracranial large and giant aneurysms. Acta Neurochir Suppl. 2021; 132:19–26.

6. Hamilton M, Kraus G, Daspit P, Spetzler R. Giant aneurysm of the vertebrobasilar trunk. In : Awad I, Barrow D, editors. Giant Intracranial Aneurysms. American Association of Neurological Surgeons;1995. p. 197–222.
7. Molyneux A, Kerr R, Yu L, Clarke M, Sneade M, Yarnold JA, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005; Sep. 366(9488):809–17.

Fig. 1.
Axial CT at presentation showed hyperdense lesion (outlined by arrows) 49 mm in length which may represent haematoma or giant aneurysm. CT, computed tomography.
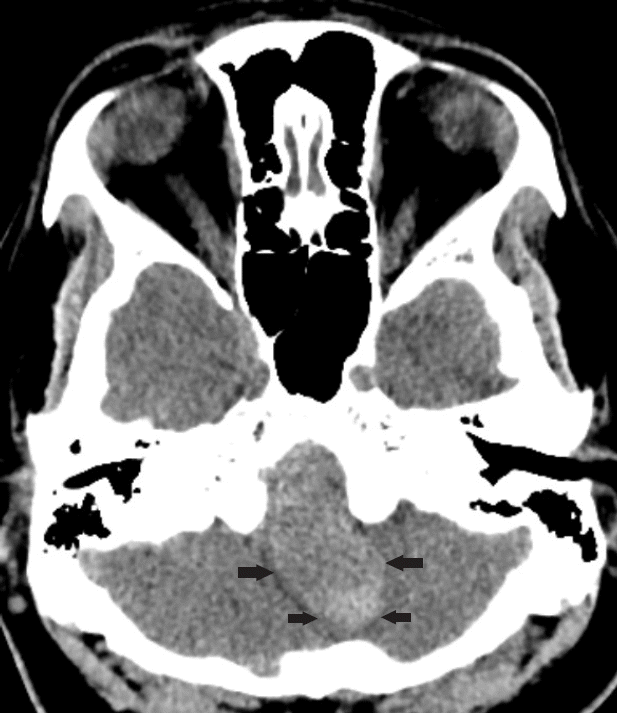
Fig. 2.
(A) Left vertebral artery angiogram AP view showed left vertebral artery giant aneurysm and PICA origin distal to aneurysm. (B) Left vertebral artery angiogram early arterial phase lateral view showed giant left vertebral artery aneurysm with PICA origin distal to it. AP, anteroposterior; PICA, posterior inferior cerebellar artery.
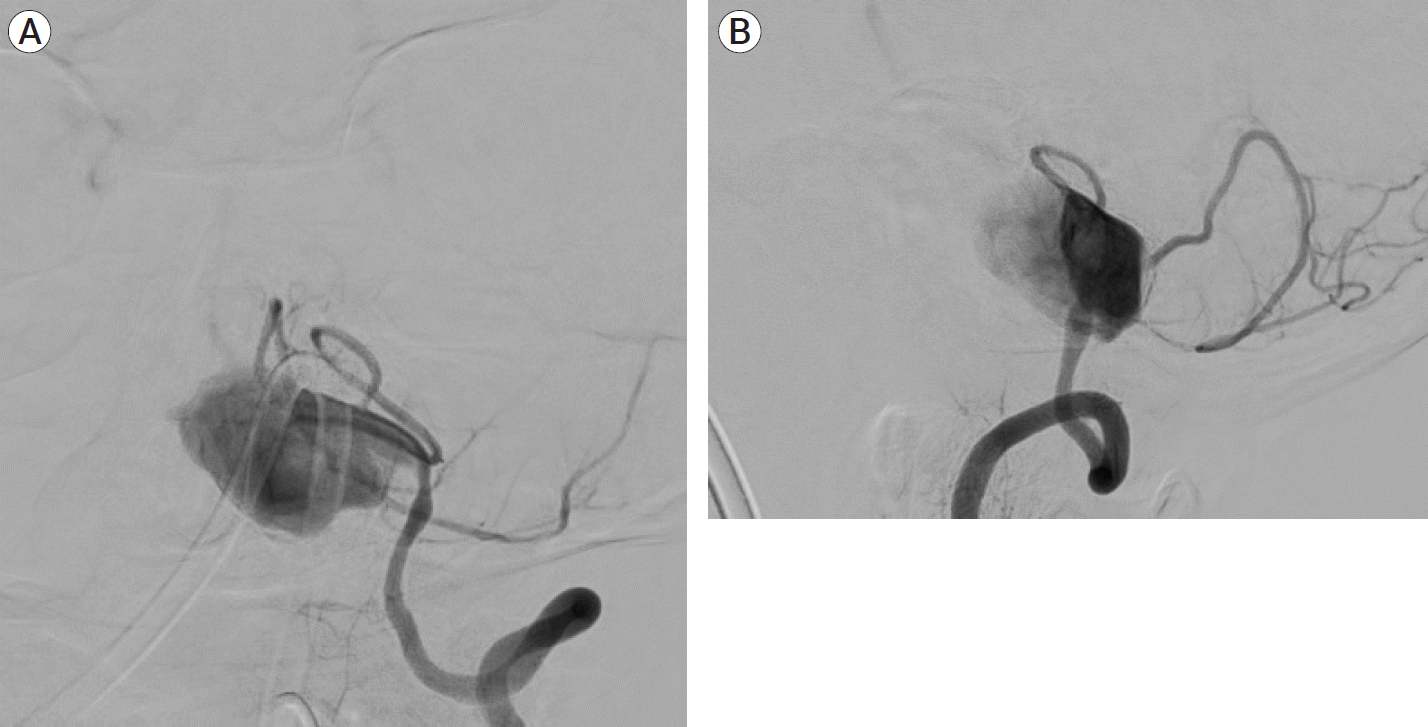
Fig. 3.
(A) Post coiling left vertebral artery angiogram AP view showed occlusion of the left vertebral artery with no filling of the aneurysm. (B) Left vertebral artery angiogram post coiling lateral view showed occlusion of the left vertebral artery with no filling of the aneurysm. AP, anteroposterior.
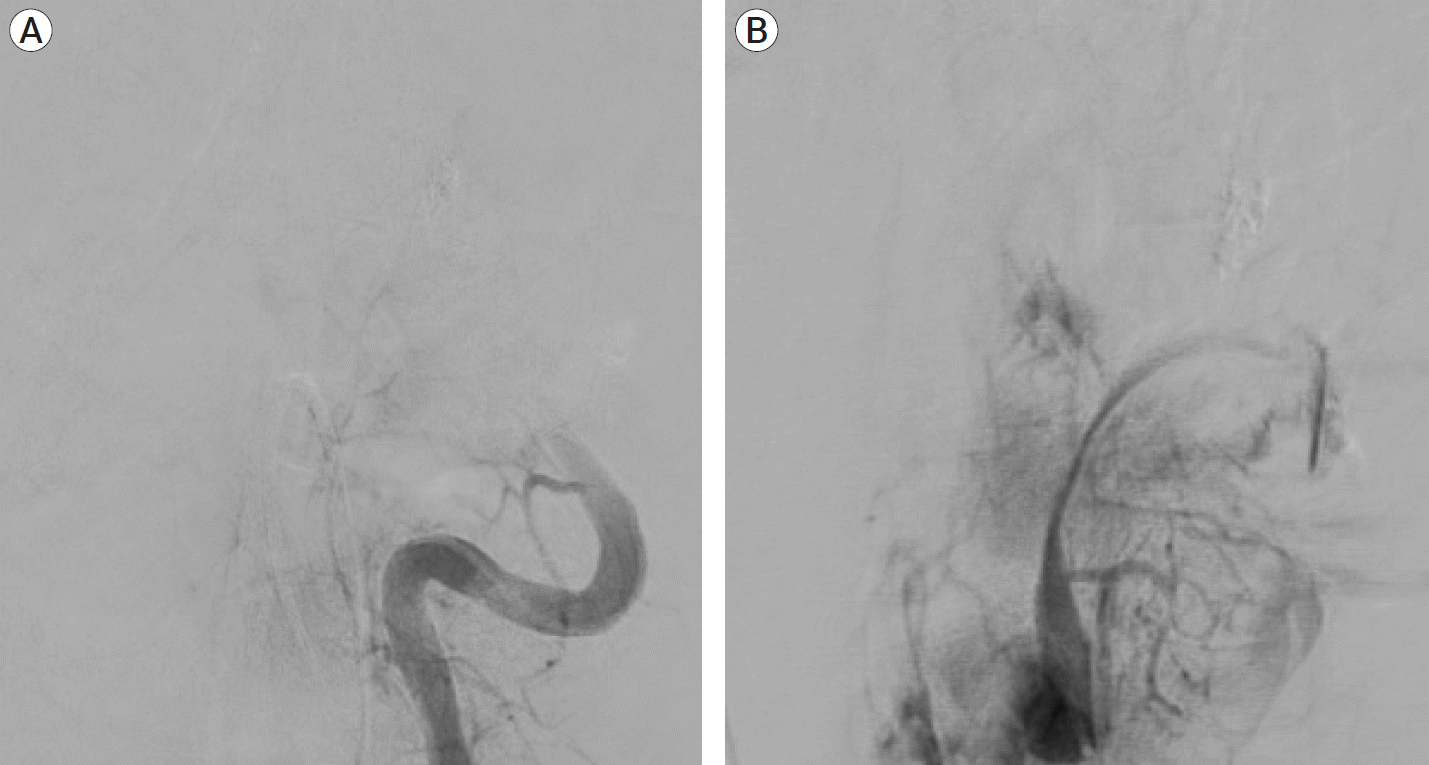
Fig. 4.
CT axial cut at the same level as Fig. 1. 3 weeks post coiling showed reduction in the size and mass effect of the posterior fossa hyperdensity with CSF spaces opening up. CT, computed tomography; CSF, cerebro-spinal fluid.
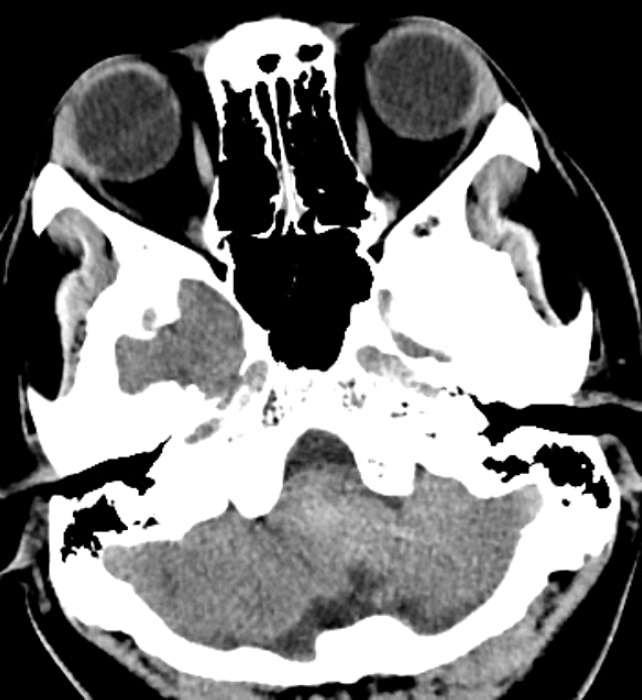
Fig. 5.
(A) CT angiogram axial view showed partially thrombosed right vertebral artery giant aneurysm with severe brainstem compression (arrows mark the outline of the aneurysm). (B) CT angiogram reconstructed AP view showed fusiform aneurysmal dilatation of the right intracranial vertebral artery with widening of the space between the two vertebral arteries due to mass effect of the partially thrombosed aneurysm. The two vertebral arteries are co-dominant. (C) CT angiogram reconstructed lateral view showed irregular fusiform aneurysmal dilatation of the intracranial right vertebral artery. CT, computed tomography; AP, anteroposterior.
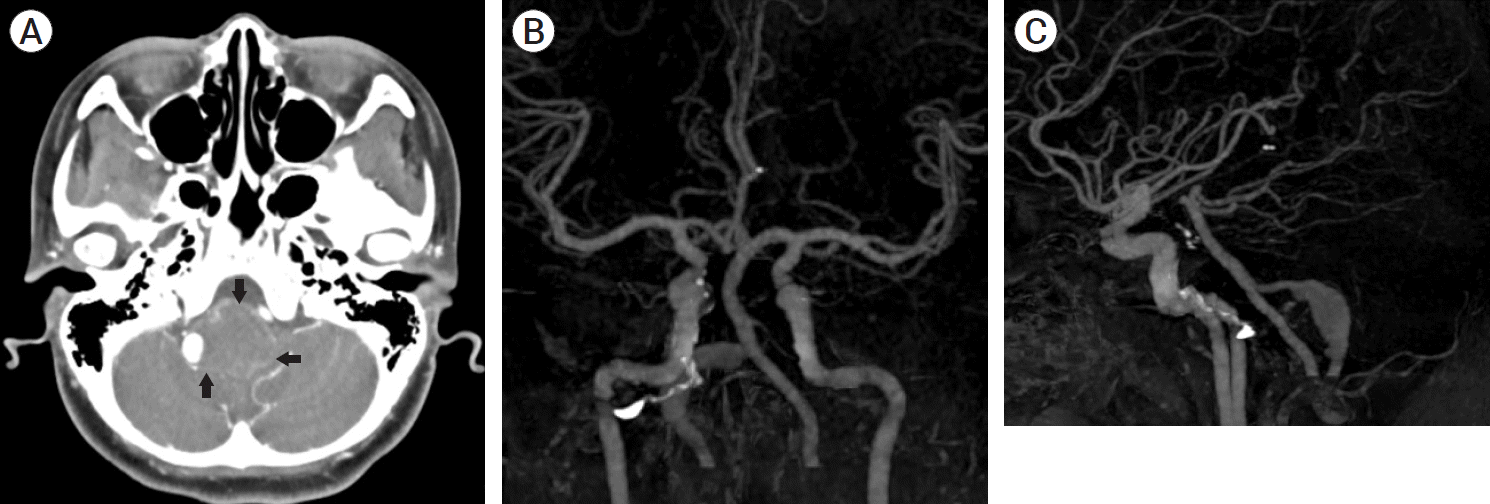
Fig. 6.
MRA at two years follow up showed no filling of right vertebral artery and resolution of aneurysm. MRA, magnetic resonance angiography.
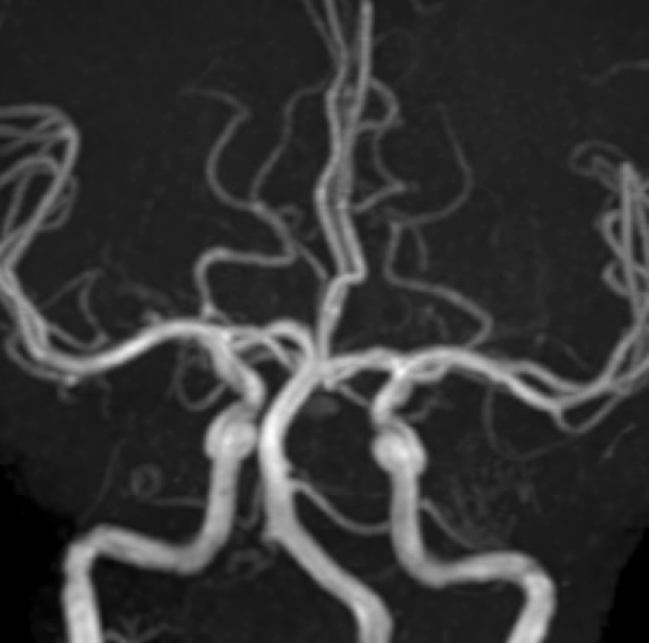
Fig. 7.
MRI coronal view showed a giant right vertebral artery aneurysm with severe brainstem compression. MRI, magnetic resonance imaging.
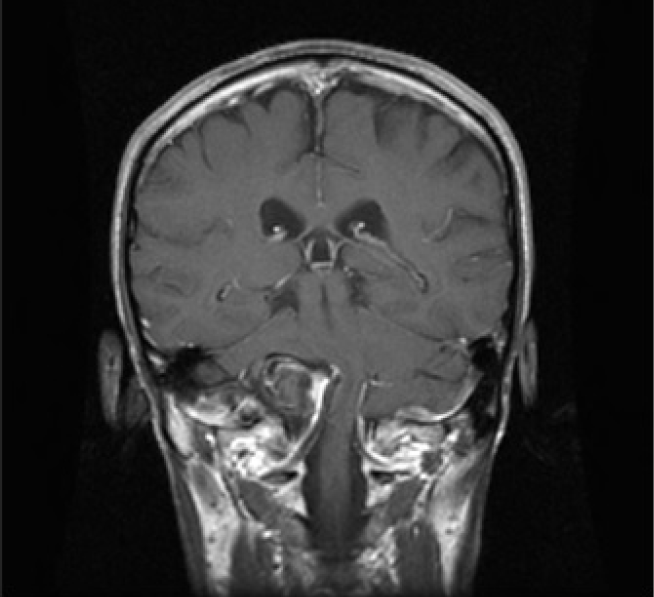
Fig. 8.
(A) Right vertebral artery angiogram lateral view showed partially thrombosed right vertebral artery giant aneurysm with PICA origin distal to it. (B) Right vertebral angiogram AP view showed partially thrombosed right vertebral artery giant aneurysm. PICA, posterior inferior cerebellar artery; AP, anteroposterior.
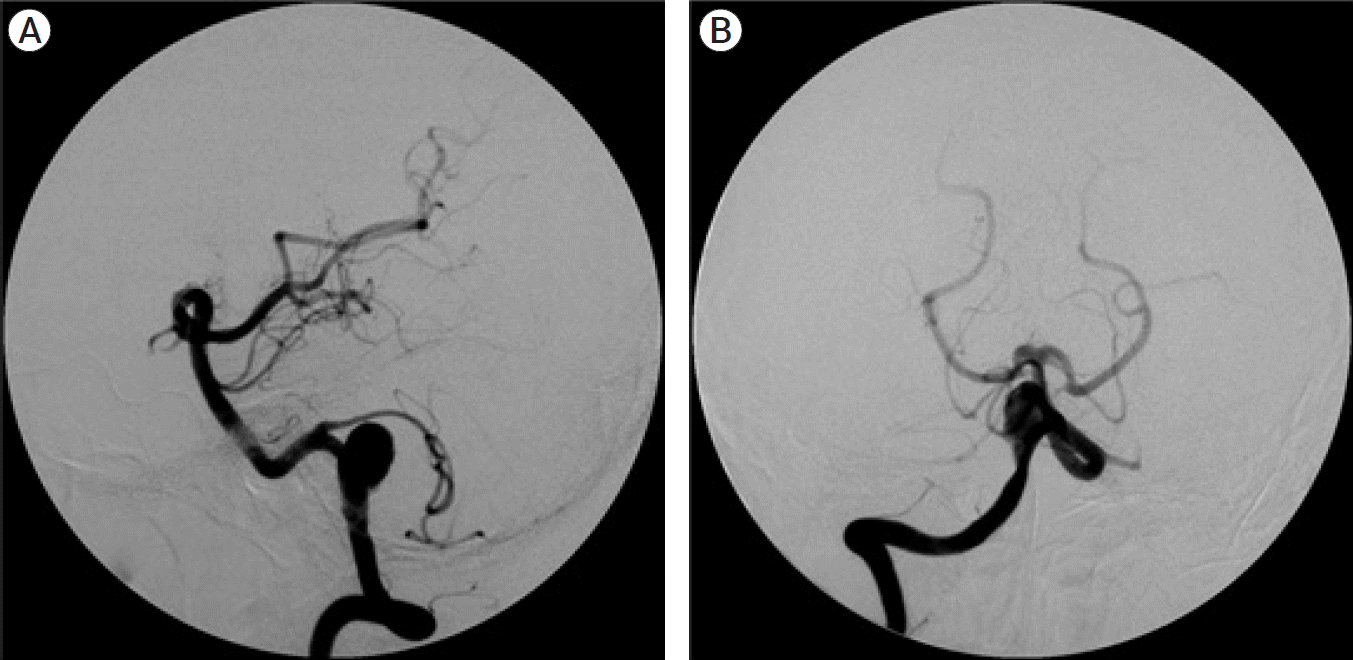
Fig. 9.
(A) Postoperative left vertebral artery angiogram lateral view showed no retrograde filling of the right vertebral artery giant aneurysm. Flow in distal right vertebral artery and right PICA was preserved. (B) Postoperative left vertebral artery angiogram AP view showed retrograde filling of distal right vertebral artery and right PICA but no retrograde filling of the original right vertebral artery giant aneurysm. PICA, posterior inferior cerebellar artery; AP, anteroposterior.
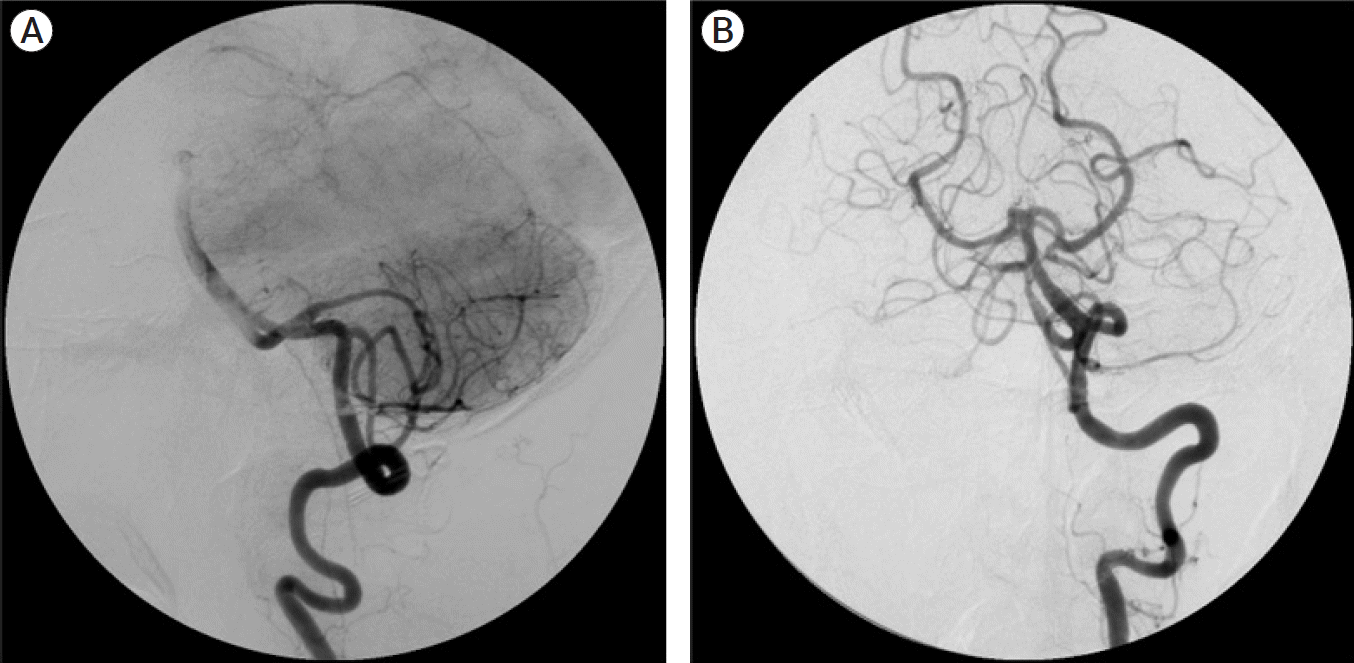
Fig. 10.
(A) Left vertebral artery angiogram AP view showing left vertebral artery giant aneurysm. (B) Left vertebral artery angiogram lateral view showing a giant left vertebral artery aneurysm distal to the PICA origin. AP, anteroposterior; PICA, posterior inferior cerebellar artery.
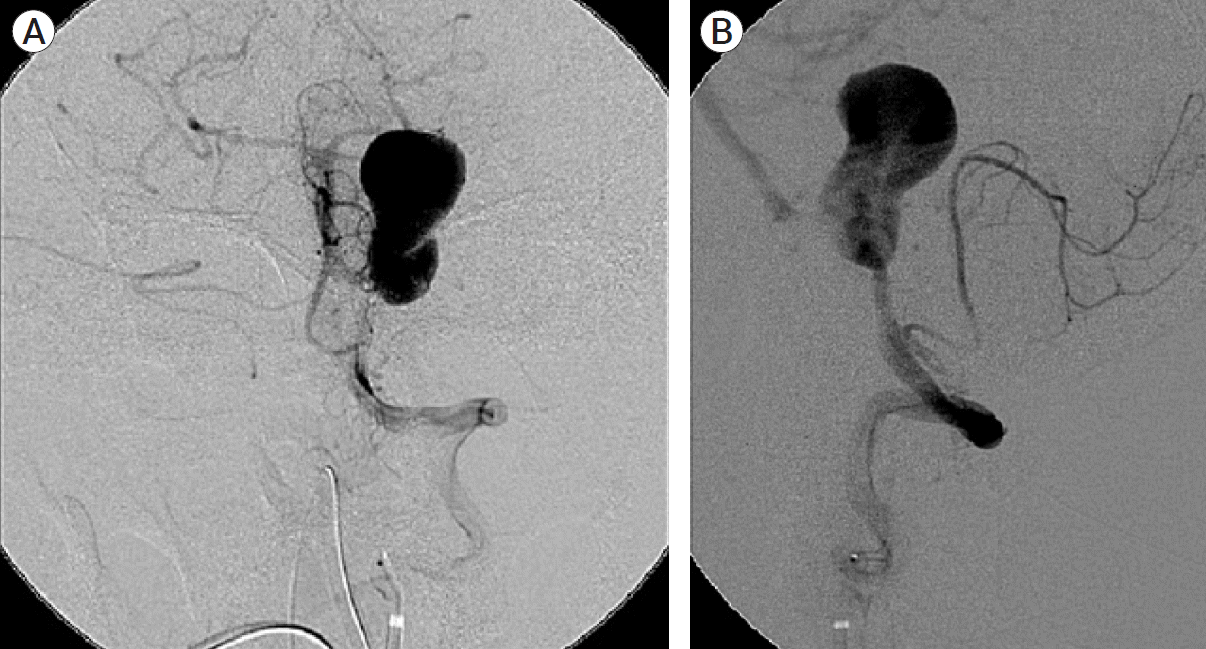
Fig. 11.
(A) Left vertebral artery angiogram AP view post coil occlusion showed no filling of the giant aneurysm. Flow in PICA was preserved. (B) Left vertebral artery angiogram lateral view post coil occlusion showed no filling of the giant aneurysm. Flow in PICA was preserved. AP, anteroposterior; PICA, posterior inferior cerebellar artery.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download