Abstract
Objective
Adrenocorticotropic hormone (ACTH) and β-endorphin are pituitary neuro-peptides released by acute stress. We determined why the prognosis of patients with subarachnoid hemorrhages (SAH) due to aneurysmal rupture is not always dependent on the Hunt–Hess grading system (HHS) and delta-National Institutes of Health Stroke Scale (NIHSS), while studying endogenous neuropeptides, including ACTH and β-endorphin.
Methods
We analyzed blood samples collected from patients with SAH (SAH group; n=37) and those with unruptured intracranial aneurysms (control group; n=37). Blood sampling was performed before any procedure or chemical agents administration. The results of ACTH and β-endorphin measurements were compared using the delta-NIHSS and HHS. The data were analyzed using descriptive statistics, independent samples t-tests, and Pearson’s correlations.
Results
Of the 18 patients with low-grade HHS, 13 had low delta-NIHSS and five showed high delta-NIHSS. Of the 19 patients with high-grade HHS, the delta-NIHSS was ≥14 in the other five patients. ACTH concentration was high (497.3 pg/mL) in five patients with high-grade HHS and high delta-NIHSS. β-endorphin concentration was high (159.7 pg/mL) in 13 patients with low-grade HHS and low delta-NIHSS.
Conclusions
High ACTH levels in patients with massive bleeding and poor neurological status suggests increasing ACTH secretion in response to bleeding stress, which may aggravate neurological status. Contrary to ACTH, high β-endorphin levels in patients with low-grade HHS implied the involvement of additional factors in predicting fair outcomes related to low delta-NIHSS. These results may provide insight into the varying prognostic potential of HHS in SAH patients.
Cerebral aneurysms occur in approximately 1 in 50 people, and its prevalence is widespread. However, they rupture at a rate of 8-10/100,000 per year; 15% patients with aneurysm rupture die before arriving at the hospital, and about 25% patients die in the hospital [6]. Of the patients who survive and receive treatment, 66% have severe permanent disabilities. Although cerebral aneurysms have been extensively studied, complete understanding of cerebrovascular constriction, which is considered as a major cause of death after cerebral aneurysm rupture, is lacking. Subarachnoid hemorrhages (SAHs) are known to be caused by the rupture of a cerebral aneurysm, resulting in the constriction of cerebrovascular blood vessels; SAHs have a profound effect on the neurological symptoms of patients at the time of bleeding and the prognosis after treatment. Therefore, Fisher grading and Hunt–Hess grading system (HHS), both of which evaluate SAHs and neurological symptoms at the time of cerebral aneurysm rupture, are essential measurements to determine the prognosis of patients [2,4,15]. However, patients without poor neurological symptoms or level of consciousness, despite a large SAH, are often encountered clinically; contrarily, patients also present with poor neurological symptoms and level of consciousness despite having a small SAH. Therefore, it is considered that the HHS and Fisher grades, along with other currently unknown factors, affect the prognosis of these patients.
Cortical influences via the limbic system, and possibly via the locus coeruleus (LC), augment the release of corticotropin-releasing hormone (CRH) during emotional stress. In contrast, peripheral input by pain and other sensory impulses to the LC stimulates the noradrenergic neurons located here via α-adrenergic receptors; these neurons project their axons to the CRH neurons [7]. A muscarinic cholinergic receptor is interposed between the α-receptors and nitric oxidergic interneurons; these interneurons release nitric oxide to promote CRH release by activating cyclic guanosine monophosphate, cyclooxygenase, lipoxygenase, and epoxygenase [13,14]. Vasopressin release during stress may have a similar mechanism. Vasopressin augments the release of CRH from the hypothalamus and enhances its action on the pituitary [9]. CRH exerts a positive ultrashort feedback loop to stimulate its own release during stress, possibly augmented by stimulating the LC noradrenergic neurons, the axons of which project into the paraventricular nucleus [9].
Adrenocorticotropic hormone (ACTH) and β-endorphin are neuropeptides derived from a common precursor, and released from the pituitary into the circulation in response to acute stress [10]. However, it is known that the stress stimulations that cause the secretion of ACTH and β-endorphin are different; β-endorphin is secreted from the A10 region of the brain, mainly as a physiological response to stimulation—such as psychological stress or anxiety, and it is stipulated that the secretion of ACTH requires a more complex response [8,11,12]. Indeed, in chronic stress, sustained activation of the sympatho-adreno-medullary system sensitizes the cortex to the action of CRH and can even activate the cortex directly. Therefore, continued activation of the sympatho-adreno-medullary system can contribute to increased adrenal corticosteroid production during chronic stress, although the elevated plasma levels of cortical hormone inhibit the pituitary response to CRH and diminish the release of further CRH into the hypophyseal portal vessels [12].
Compare with relaxed individuals, stressed individuals secrete a greater amount of β-endorphin, which inhibits the role of stress hormones, and this effect is difficult to overcome even under great stress. Moreover, β-endorphin acts on the Na-K pumps on the nerve cells and blood vessel membranes to induce stability of the pain system and nerve cell membranes. Therefore, β-endorphins can act even after SAH occurs and can be secreted under extreme stress.
This study compares the concentrations of ACTH and β-endorphin in blood samples obtained from individuals with SAH and those with unruptured aneurysms (controls). We also analyzed the relationship between these concentrations and patient prognoses using the delta-National Institutes of Health Stroke Scale (NIHSS) score, which is calculated by subtracting the NIHSS score at hospitalization from the NIHSS score at patient discharge [5], to predict the prognosis of patients with SAH.
We collected blood samples from 37 patients diagnosed with SAH by ruptured aneurysm in the emergency room between March 2018 and July 2019. Blood samples were collected randomly from 37 patients diagnosed with unruptured intracranial aneurysms who underwent transfemoral cerebral angiography during the same period. All blood sampling procedures were conducted after obtaining informed consent from the patients or their families. All the collected blood samples were centrifuged to separate plasma and frozen in the Human Resource Bank until the blood collection for all patients was completed. After the blood collection was completed, ACTH concentration in each sample was measured using an ACTH Immunoradiometric assay kit (IRMA; BRAHMS, Hennigsdorf, Germany) and the output was analyzed using an r-counter (COBRA 5010 series Quantum, PACKARD, Meriden, USA). β-endorphin concentration was measured by enzyme-linked immunosorbent assay (ELISA) using a QuickDetectTM β-endorphin (Human) ELISA Kit (Bio Vision, Milpitas, CA, USA), and the output was analyzed using a microplate reader (VERSA Max, Molecular Devices, San Jose, CA, USA). All analyses were performed at Global Clinical Central Lab (GCCL, Yongin-si, Gyeonggi-do, Korea).
In addition, neurological evaluation and the classification of the initial HHS grade and delta-NIHSS score were performed in all patients. We calculated the delta-NIHSS score by subtracting the NIHSS score at hospitalization from the NIHSS score at patient discharge to predict the prognosis of patients with SAH.
HHS grades were classified as either “low” (grades I and II) or “high” (grades III, IV, and V). Delta-NIHSS score was defined as “low” (score <1.51; average of calculated delta-NIHSS) or “high” (score >1.51). The data were analyzed using descriptive statistics, independent samples t-tests (SPSS version 25, IBM Corporation, Armonk, NY, USA; p<0.05), and Pearson’s correlations.
The characteristics of SAH patients and those in the control group showed no significant differences in terms of age and sex ratio (Table 1). ACTH level was markedly different between the SAH and control groups (Fig. 1). However, β-endorphin levels did not show a prominent difference (Fig. 1). The delta-NIHSS score was relatively high in patients with high ACTH levels, which could be considered to indicate a significant association (Fig. 2A); however, estimating a significant association between delta-NIHSS scores and β-endorphin levels was difficult (Fig. 2B). In both the groups, the higher the delta-NIHSS score (which indicates poor prognosis), the higher the ACTH level (Fig. 3). Despite no significant association between HHS grades and delta-NIHSS scores (Fig. 4A), high ACTH levels were observed in patients with high delta-NIHSS scores (>1.51) when compared to those with low delta-NIHSS scores (<1.51) (Fig. 4C). Seemingly, high ACTH levels correlated with poor prognosis in patients with SAH; however, these results did not show any statistically significant difference.
Moreover, in the low-grade HHS group, patients with poor prognosis—those who had high delta-NIHSS scores—did not have high ACTH levels when compared to patients with low delta-NIHSS scores (Fig. 4B). In addition, in the high-grade HHS group, each patient with a fair prognosis who had low delta-NIHSS scores also had low ACTH levels when compared to the patients with high delta-NIHSS scores (Fig. 4B); however, these results were not statistically significant (p=0.377). The average concentration of ACTH was higher in the low delta-NIHSS score than in the high delta-NIHSS groups when assessed among those with low-grade HHS; contrarily, ACTH concentration was higher in the high delta-NIHSS score group than in the low delta-NIHSS score groups when assessed among those with high-grade HHS (Table 2, Fig. 5A). In addition, among the patients with low-grade HHS, β-endorphin levels were higher in the low delta-NIHSS score groups than in the high delta-NIHSS score groups (Table 3). Although not statistically significant, β-endorphin levels were relatively high in the patient group with a fair prognosis (Fig. 5B).
It is well known that CRH, a peptide hormone that stimulates both the synthesis and the secretion of ACTH in the corticotropin-producing cells (corticotrophs) of the anterior pituitary gland, increases β-endorphin (beta-end like) concentration in cerebrospinal fluid of rats with vasospasm following subarachnoid hemorrhage [3]. However, in previous study, SAH severity did not affect cortisol concentrations, possibly indicating relative pituitary-adrenal insufficiency in patients with more severe bleeding [1].
We expect a significant difference in ACTH and β-endorphin levels in patients with SAH, depending on their prognosis. We also expect a good or fair prognosis in patients with SAH with relatively high ACTH and β-endorphin levels.
In this study, no significant correlation was observed between ACTH and β-endorphin levels and patient prognosis. However, in the low-grade HHS group, the ACTH level was high in the poor prognosis group, and β-endorphin levels were high in the fair prognosis group.
The study results confirmed that the secretion of ACTH was significantly increased, unlike that of β-endorphin, under the stressful situation of SAH among patients with high-grade HHS; this increased secretion was estimated to be related to poor outcomes when compared with the delta-NIHSS score. In addition, even in the high-grade HHS group in which poor prognosis can be predicted classically, the higher the ACTH levels, the higher the delta-NIHSS score, considering that the higher the ACTH level, the higher the likelihood of a poor prognosis.
However, the results were contradictory in the low-grade HHS group. Patients in the low-grade HHS group had higher ACTH levels and higher β-endorphin levels than those in the high-grade HHS group. In the high-grade HHS group, β-endorphin level was relatively higher in those with a fair prognosis than those with a poor prognosis. These results suggest that a fair prognosis can be predicted in patients with high ACTH levels when the β-endorphin level is relatively high. However, the interpretation of this result should be analyzed carefully.
In our study, a positive correlation was seen between ACTH levels and the prognosis of patients with SAH, and it is estimated that higher ACTH levels can predict poor prognosis in patients with SAH. Additionally, SAH patients with low-grade HHS who have high β-endorphin levels could be predicted to have a fair prognosis; however, no statistically significant correlation was seen for this prediction.
In the low-grade HHS group, ACTH level was higher in the good or fair prognosis group than in the poor prognosis group. Moreover, in the high-grade HHS group, ACTH level was higher in those with a poor prognosis than in those with a fair prognosis. These results suggest no association between ACTH levels and patient prognosis.
However, this study to compare ACTH and β-endorphin levels was performed with small patient group. To conclusively clarify the correlation, further multicentric studies with relatively large patient populations are needed.
If further studies are conducted on a large number of patients, poor prognosis could be predicted in those with high ACTH levels, and when β-endorphin levels are high in this patient group, a relatively fair prognosis may be predicted.
Higher ACTH levels in patients with a large SAH and poor neurological status suggest that ACTH is increasingly secreted in response to physical stress occurring during bleeding, which may aggravate patients’ neurological status. The higher the initial ACTH concentration measured in patients with high-grade HHS, the worse the prognosis—this prognosis is related to a high NIHSS score. Contrary to the role of ACTH, high levels of β-endorphin in patients with low-grade HHS suggests the potential involvement of additional factors that could predict fair outcomes related to low NIHSS scores. These data could provide some clue of resolution for the dissociation of prognosis according to HHS grades in patients with aneurysmal ruptures.
REFERENCES
1. Bendel S, Koivisto T, Ruokonen E, Rinne J, Romppanen J, Vauhkonen I, et al. Pituitary-adrenal function in patients with acute subarachnoid haemorrhage: a prospective cohort study. 2008; Oct. 12(5):R126.

2. Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980; Jan. 6(1):1–9.

3. Henryk S, Jośko J, Jedrzejowska-Szypułka H, Jarzab B, Döhler KD. Corticotropin releasing hormone (CRH) increases beta-endorphin (beta-end like) concentration in cerebrospinal fluid of rats with vasospasm following subarachnoid hemorrhage. J Physiol Pharmacol. 1999; Sep. 50(3):419–28.
4. Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968; Jan. 28(1):14–20.

5. Kim YD, Song D, Kim EH, Lee KJ, Lee HS, Nam CM, et al. Long-term mortality according to the characteristics of early neurological deterioration in ischemic stroke patients. Yonsei Med J. 2014; May. 55(3):669–75.

7. Makino S, Shibasaki T, Yamauchi N, Nishioka T, Mimoto T, Wakabayashi I, et al. Psychological stress increased corticotropin-releasing hormone mRNA and content in the central nucleus of the amygdala but not in the hypothalamic paraventricular nucleus in the rat. Brain Res. 1999; Dec. 850(1-2):136–43.

8. Marschall K, Schlesinger MD, Turndorf H, Puig MM. Beta-endorphin and ACTH levels in the perioperative period. Gen Pharmacol. 1989; 20(4):399–402.

9. McCann SM, Antunes-Rodrigues J, Franci CR, Anselmo-Franci JA, Karanth S, Rettori V. Role of the hypothalamic pituitary adrenal axis in the control of the response to stress and infection. Braz J Med Biol Res. 2000; Oct. 33(10):1121–31.

10. Pilozzi A, Carro C, Huang X. Roles of beta-Endorphin in Stress, Behavior, Neuroinflammation, and Brain Energy Metabolism. Int J Mol Sci. 2020; Dec. 22(1):
11. Schedlowski M, Flüge T, Richter S, Tewes U, Schmidt RE, Wagner TO. Beta-endorphin, but not substance-P, is increased by acute stress in humans. Psychoneuroendocrinology. 1995; 20(1):103–10.

12. Sprouse-Blum AS, Smith G, Sugai D, Parsa FD. Understanding endorphins and their importance in pain management. Hawaii Med J. 2010; Mar. 69(3):70–1.
13. Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009; Jun. 10(6):397–409.

Fig. 1.
Comparison of ACTH and β-endorphin concentrations between the SAH and control groups (p<0.05 considered statistically significant). ACTH, adrenocorticotropic hormone; SAH, subarachnoid hemorrhages.
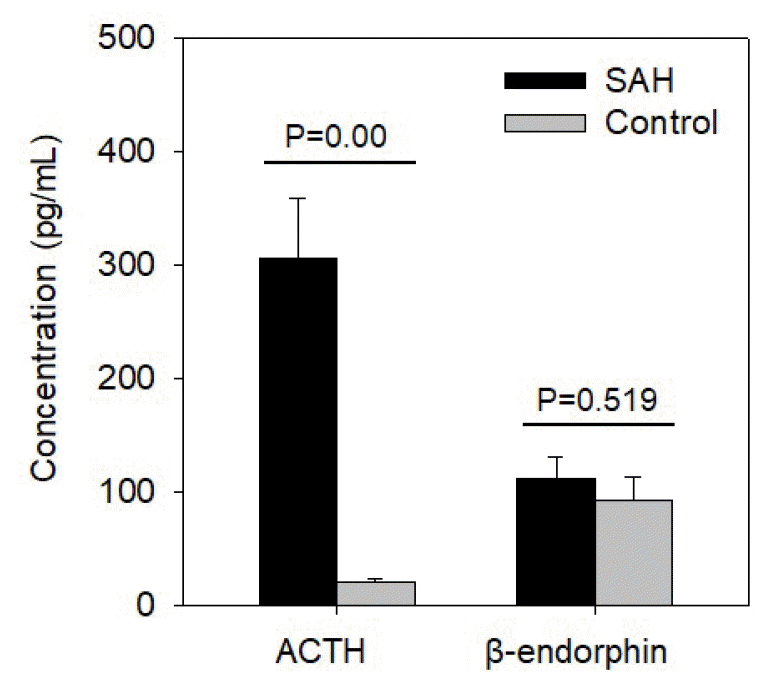
Fig. 2.
The concentration of (A) ACTH and (B) β-endorphin depending on delta-NIHSS scores (Correlation is expressed by Pearson’s correlation coefficient [r] as appropriate, with p<0.05 indicating statistical significance). ACTH, adrenocorticotropic hormone.
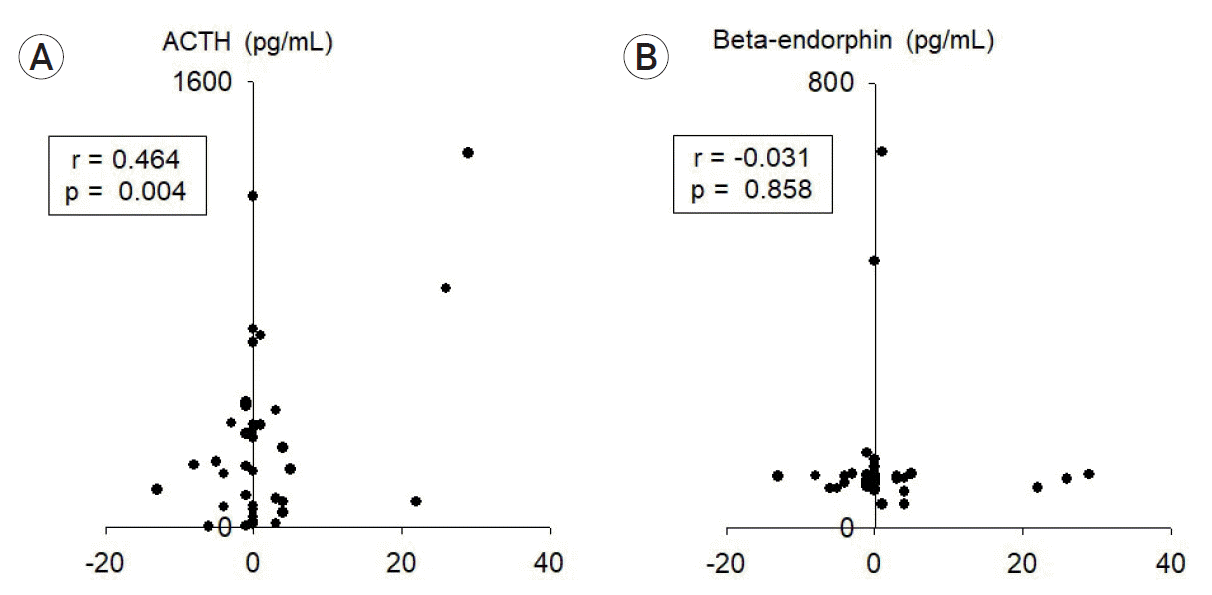
Fig. 3.
Scatter plot of ACTH (pg/mL) and β-endorphin (pg/mL) with delta-NIHSS score in the (A) low-grade HHS and (B) high-grade HHS groups (correlation is expressed by Pearson’s correlation coefficient [r] as appropriate, with p<0.05 indicating statistical significance). ACTH, adrenocorticotropic hormone; HHS, Hunt–Hess grading system; NIHSS, National Institutes of Health Stroke Scale.
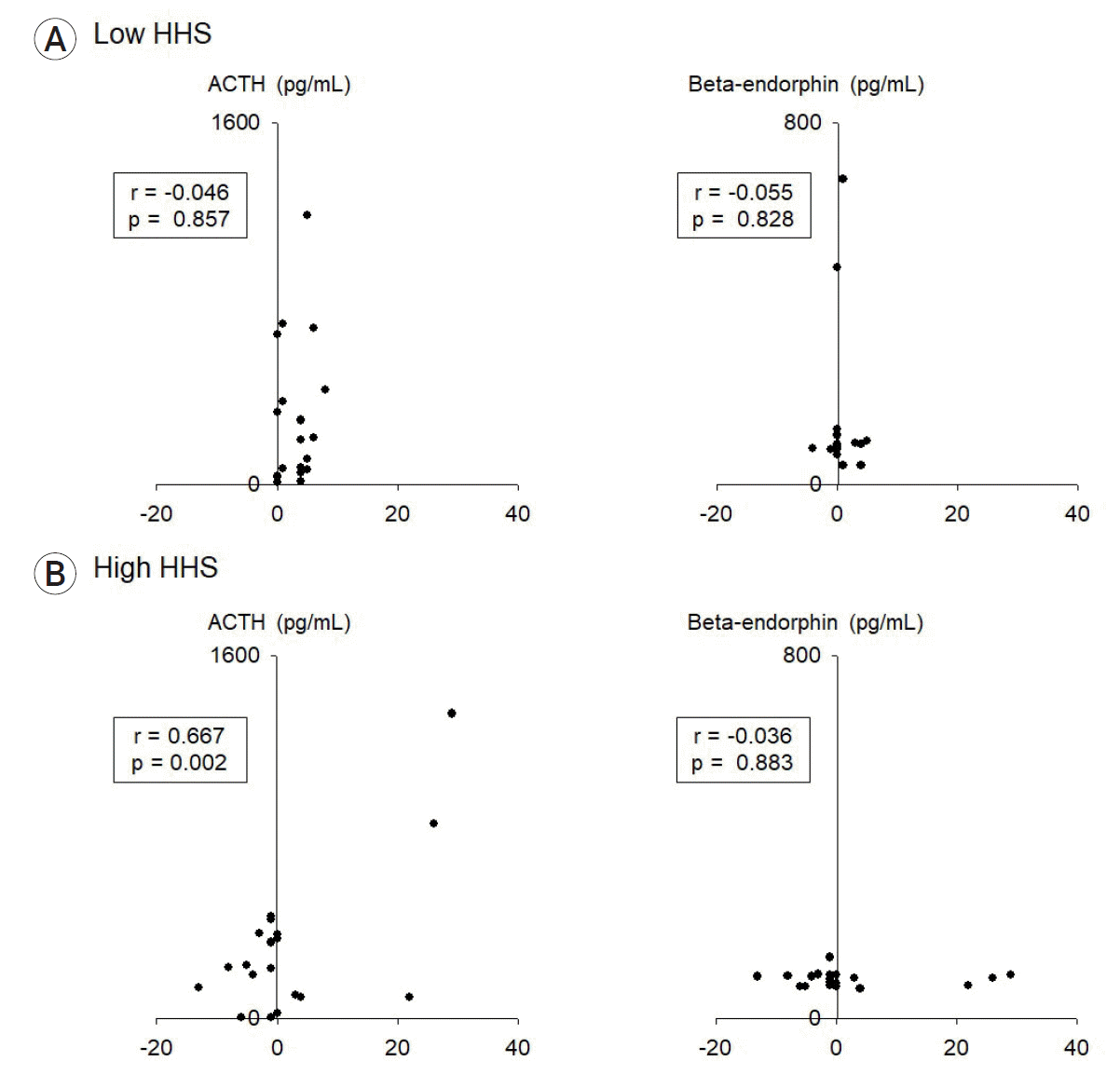
Fig. 4.
(A) The distribution of SAH patients by HHS grades and delta-NIHSS scores. (B) Concentration of ACTH and β-endorphin divided by HHS grades and delta-NIHSS scores. (C) Comparison of ACTH and β-endorphin concentrations stratified by the delta-NIHSS score (p<0.05 indicates statistical significance). ACTH, adrenocorticotropic hormone; SAH, subarachnoid hemorrhages; HHS, Hunt–Hess grading system; NIHSS, National Institutes of Health Stroke Scale.
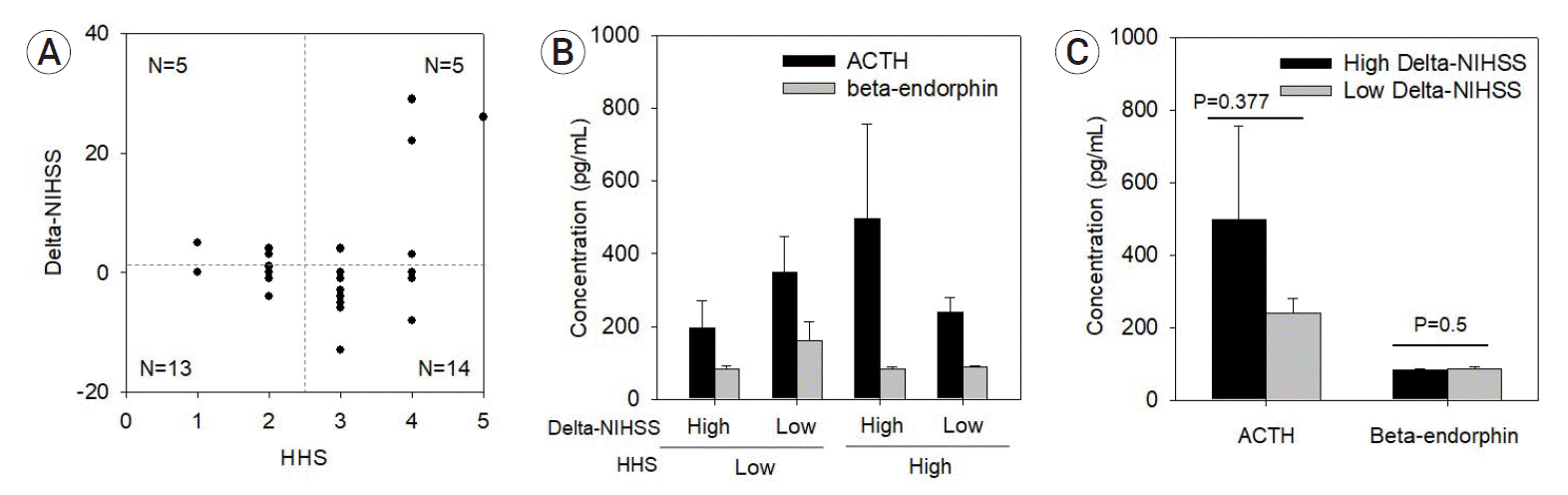
Fig. 5.
The concentration of (A) ACTH and (B) β-endorphin divided by HHS grades and delta-NIHSS scores (p<0.05 indicates statistical significance). ACTH, adrenocorticotropic hormone; HHS, Hunt–Hess grading system; NIHSS, National Institutes of Health Stroke Scale.
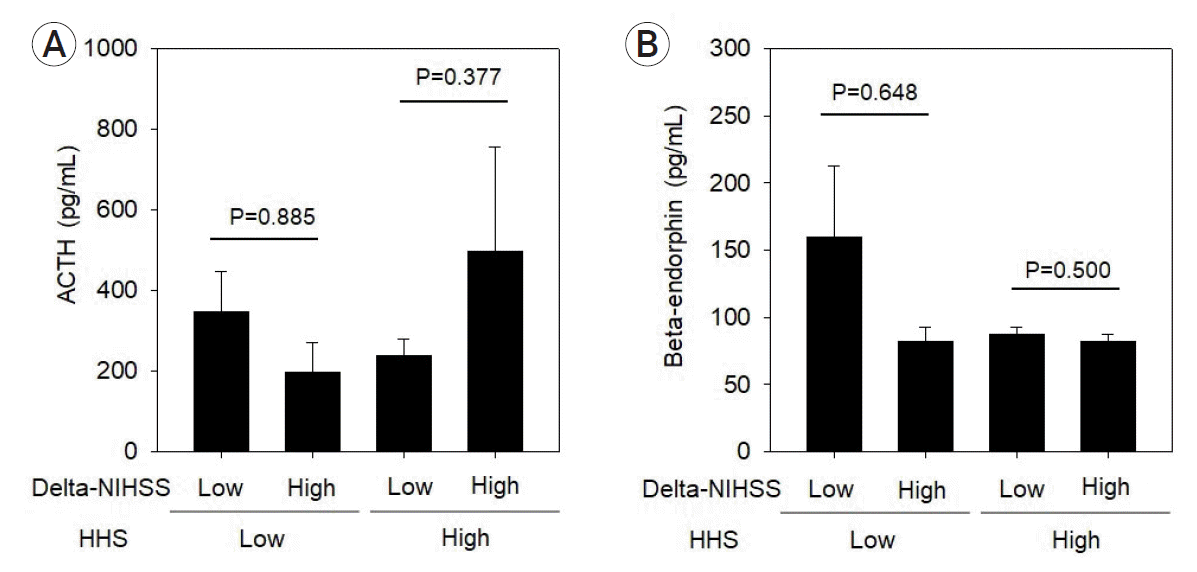
Table 1.
Patient characteristics at baseline
| Characteristic | SAH group (N=37) | Control group (N=37) |
|---|---|---|
| Age (yr) | 65.8±14.6 | 64.6±9.0 |
| Male sex-no. (%) | 13 (35.1) | 10 (26.3) |
Table 2.
The average concentration of ACTH and β-endorphin divided by HHS and delta-NIHSS
Table 3.
The average concentration of ACTH and β-endorphin between the SAH and control groups




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download