Abstract
Moyamoya syndrome (MMS) associated with hyperthyroidism, such as Graves’ disease, is a rare condition that causes ischemic stroke with thyrotoxicity. A 43-year-old woman with symptoms of right hemiparesis was admitted. Brain magnetic resonance imaging revealed a small cerebral infarction in the left frontal lobe. Cerebral angiography revealed multi-vessel intracranial occlusive disease. Several days later, neurologic deterioration and aggravation of cerebral infarction developed due to a thyroid storm. A thyroid function test revealed the following: thyroid-stimulating hormone (TSH) <0.01 μunits/mL (reference range, 0.55–4.78 μunits/mL); triiodo-thyronine >8.0 ng/mL (reference range, 0.77–1.81 ng/mL); free thyroxine (T4) of 9.47 pmol/L (reference range, 11.4–22.6 pmol/L); and TSH receptor antibody of 37.10 U/L (reference range, 0–10 U/L). For thyroid storm management, we initiated treatment with methimazole, Gemstein’s solution, and hydrocortisone. Finally, the thyroid disease was controlled, and neurologic deficits improved. We describe a case of acute cerebral infarction combined with a thyroid storm in a patient with Moyamoya syndrome and Graves’ disease. Hyperthyroidism such as Graves’ disease should be considered in the differential diagnosis for patho-etiologic mechanisms associated with MMS. A cerebrovascular disease with a thyroid storm can lead to severe mortality and morbidity. Prompt diagnosis and strict treatment are important.
Moyamoya disease (MMD) is an uncommon, chronic cerebrovascular disease in which stenosis–occlusion of the bilateral internal carotid arteries follows the development of characteristic Moyamoya vasculature at the base of the brain [24]. People with a well-known related disorder are classified as having Moyamoya syndrome (MMS), whereas those with an idiopathic disease and no known risk factors are classified as having MMD. The well-recognized associated diseases are sickle cell anemia, Down syndrome, neurofibromatosis, and rarely, hyperthyroidism such as Grave’s disease (GD) [19]. Moyamoya vasculopathy combined with hyperthyroidism is an uncommon condition that causes ischemic stroke with thyrotoxicity. A thyroid storm, an endocrine condition associated with uncontrolled hyperthyroidism, in a patient with underlying Moyamoya syndrome (MMS) can lead to increased morbidity and mortality rates. Also, depending on the order of the disease in which Thyrotoxicosis, acute infarction, and MMD were diagnosed, the hospitalization department for endocrinology, neurology, and neurosurgery may vary. It is considered relatively difficult for neurosurgeons to experience. We report a case of acute cerebral infarction combined with a thyroid storm in a patient with MMS and GD.
A 43-year-old woman was admitted to a local hospital with weakness in her right side. Diffusion magnetic resonance imaging (MRI) showed a small cerebral infarction in the left frontal lobe (Fig. 1A). Conservative management was done with hydration and antiplatelet therapy. However, three days later, her neurologic condition deteriorated. Her right-sided weakness was aggravated with newly developed left-sided weakness and dysarthria. Also, her level of consciousness became stuporous. She was transferred to our institution.
On arrival at our institution, her Glasgow Coma Scale (GCS) score was 9 (E2V2M5). Her motor grades were upper extremities III/I and lower extremities IV/III. Her blood pressure was 175/80 mmHg with a heart rate of 150 beats/min, and her electrocardiography (ECG) indicated atrial fibrillation with a rapid ventricular response (RVR). A follow-up MRI, including a diffusion and perfusion sequence, was conducted. Compared with the previous MRI findings, the infarction territory was markedly increased (Fig. 1B). Cerebral angiography was subsequently performed. It revealed occlusion of both distal ICAs, with the characteristics of Moyamoya vessels and poor collateral flow in both frontal lobes. These angiographic findings were most compatible with a diagnosis of MMD (Fig. 2). The patient underwent conservative treatment, including hydration and antiplatelet therapy in the intensive care unit. A few days later, she had a sudden seizure. Her vital signs indicated high blood pressure and tachycardia, with a fever of up to 38.5℃. Electrocardiography revealed atrial fibrillation with RVR, and chest radiography showed cardiomegaly. Her seizure was controlled by an anticonvulsant. Simultaneously, rhythm and rate control therapy with amiodarone and beta-blockers was used to stabilize the blood pressure and heart rate and achieve chemical de-fibrillation. An interview with her family revealed that she had a history of hyperthyroidism. A thyroid function test was done, and it revealed the following: thyroid-stimulating hormone (TSH)<0.01 µunits/mL (reference range, 0.55–4.78 µunits/mL); triiodo-thyronine>8.0 ng/mL (reference range, 0.78–1.81 ng/mL); free thyroxine (T4) of 9.47 pmol/L (reference range, 11.4–22.6 pmol/L); and TSH receptor antibody of 37.10 U/L (reference range, 0–10 U/L). By consultation with an endocrinologist, we diagnosed a thyroid storm. The thyroid storm score (Burch and Wartofsky score) was 85 points. We initiated management of the thyroid dysfunction with methimazole, Gemstein’s solution, and hydrocortisone. The patient was transferred to the general ward after three weeks of intensive care, and her neurological condition improved with rehabilitation. At discharge, her level of consciousness was drowsy, and her motor power grades were upper extremities III/II and lower extremities III/II. Six months later, her level of consciousness was alert. Her mini-mental state examination (MMSE) score was 27/30. Her motor grades were upper V/IV and lower V/V. The modified Barthel active daily living (ADL) index was 91/100. Medications for GD and epilepsy were continued.
GD is unusually complicated by Moyamoya vasculopathy, resulting in an ischemic stroke with thyrotoxicity [11,17]. The association of GD with MMD was first reported by Kushima et al., who documented two cases of patients presenting with clinical symptoms of thyrotoxicosis, followed by strokes [10]. Until recently, approximately 50 cases of co-occurring GD and MMD had been described in the literature [20].
GD, known as a toxic, diffuse goiter, is an autoimmune disease that affects the thyroid and is the most common cause of hyperthyroidism [2]. The Irish surgeon Robert Graves first described the condition in 1835 [15]. It is affected by thyroid-stimulating immunoglobulin (TSI), which is similar to thyroid-stimulating hormone (TSH). The TSI antibodies induce the thyroid gland to secrete excess thyroid hormones. GD occurs in about 3% of females and 0.5% of males [2]. It most often begins between the ages of 40 and 60 but can begin at any age. The definitive cause is unknown; however, it is believed to include a combination of environmental and genetic factors [12]. This disease may occur as a response to physical or emotional stress or infection [1].
Although the pathogenesis of MMD with GD is not well known, it has been suggested that the autoimmune mechanisms involved in the development of GD and overactivation of the sympathetic nervous system in a hyperthyroid state play essential roles in the occurrence of steno-occlusive lesions in the intracranial arteries [16]. Other disorders combined with MMS, including antiphospholipid syndrome, systemic lupus erythematosus, tuberculosis, leptospirosis, ulcerative colitis, and post-radiation arteritis, have well-defined autoimmune mechanisms of pathogenesis or are connected with arteritis that affects the cerebral arteries [22]. Even non-vasculitic diseases like Down syndrome, which is related to MMS, may be associated with autoimmune etiologies [9].
Our patient had a history of GD but was not taking medication for the disease, making the risk of developing a thyroid storm very high. Generally, thyroid storms are uncommon, but the complication of hyperthyroidism has high mortality and severity. A high fever (often above 40°C), fast and often irregular heartbeats, elevated blood pressure, vomiting, diarrhea, and agitation are symptoms of thyroid storms [14]. Thyroid storms are typically triggered by stressful medical conditions, such as uncontrolled hyperthyroidism, cessation of anti-thyroid drugs, surgery, trauma, infection, and diabetic ketoacidosis [18]. The pathophysiological mechanisms of thyroid storms are combinations of sharp increases in the secretion of thyroid hormones, relative adrenal insufficiency, hyperactivity of the sympathetic nervous system, and increased peripheral cellular reactions to thyroid hormones. Thyroid storms are characterized by the dysfunction of multiple organ systems, such as the gastrointestinal system, cardiovascular system, hepatic system, thermoregulatory system, and central nervous system [3], and are related to thromboembolic events. Atrial fibrillation and ventricular dysrhythmias associated with hyperthyroidism have a high risk of leading to a cardioembolic stroke [23]. Mortality is associated with shock, multiple organ failure, or disseminated intravascular coagulation (DIC) [18]. The overall mortality rate of cardioembolic strokes is between 10% and 30%. In a thyrotoxicotic state, overproduction of thyroid hormones can affect vascular reactivity and cause cerebral perfusion dysfunction. Therefore, activities related to thyrotoxicosis affect ischemic cerebrovascular symptoms in MMD [5,21]. In our study, the neurological symptoms of the patient worsened three days after the stress caused by the preceding ischemic stroke, and their neurological symptoms improved after treatment of the thyroid storm with methimazole, Gemstein’s solution, and hydrocortisone. Table 1 summarizes the clinical and radiologic data, treatment and outcomes, for similar cases reported earlier [4-8,11,13]. Jang et al. reported that a 41-year-old woman diagnosed with a thyroid storm during an acute cerebral infarction of 90 points on the Burch and Wartofsky scale. After intensive treatment for the thyroid storm, the seizures, unexplained fever, and neurologic symptoms improved [7]. Kang et al. also reported a similar case in which thyrotoxicosis aggravated the acute cerebral infarction caused by MMD [8].
Our study case is uncommon. However, prompt diagnosis and management are essential for improving the prognoses of MMD and GD patients who have experienced ischemic strokes. Ischemic cerebrovascular events can be risk factors for thyroid storms. If cerebral infarction or transient ischemic attacks develop in a patient with combined GD and MMD, treatment should be initiated immediately. Also, maintenance of a long-term euthyroid state is essential for preventing a relapse of hyperthyroidism after normal thyroid function has been restored in MMD patients. Treatment modalities for patients in our facility include oral anti-thyroid medications, antiplatelet agents, and corticosteroids. Management of GD is essential to prevent recurrence, but Moyamoya vasculopathy may progress even in controlled GD [6].
We report an uncommon case of acute cerebral infarction combined with a thyroid storm in a patient with MMS and GD. It should be noted that the simultaneous occurrence of a thyroid storm and cerebrovascular accident in MMS and GD highly elevates mortality and morbidity. Prompt diagnosis and aggressive treatment are essential for saving a patient’s life. Therefore, if neurological symptoms occur in patients with histories of combined MMD and GD, neurosurgeons should contemplate thyroid function tests for identifying thyroid storms, and aggressive treatment should be initiated.
REFERENCES
2. Burch HB, Cooper DS. Management of Graves disease: a review. JAMA. 2015; Dec. 314(23):2544–54.
3. Burch HB, Wartofsky L. Life-threatening thyrotoxicosis: thyroid storm. Endocrinol Metab Clin North Am. 1993; Jun. 22(2):263–77.

4. Golomb MR, Biller J, Smith JL, Edwards-Brown M, Sanchez JC, Nebesio TD, et al. A 10-year-old girl with coexistent moyamoya disease and Graves’ disease. J Child Neurol. 2005; Jul. 20(7):620–4.

5. Im S-H, Oh CW, Kwon O-K, Kim JE, Han DH. Moyamoya disease associated with Graves disease: special considerations regarding clinical significance and management. J Neurosurg. 2005; Jun. 102(6):1013–7.

6. Ito H, Yokoi S, Yokoyama K, Asai T, Uda K, Araki Y. Progressive stenosis and radiological findings of vasculitis over the entire internal carotid artery in moyamoya vasculopathy associated with Graves’ disease: a case report and review of the literature. BMC Neurol. 2019; Mar. 19(34):

7. Jang SA, Baek YH, Park TS, Lee KA. A case of acute cerebral infarction and thyroid storm associated with Moyamoya disease. Int J Thyroidol. 2017; 10(1):56–60.

8. Seong G-H, Bae JS, Lee J-H, Kim Y. Acute ischemic stroke in Moyamoya syndrome associated with thyrotoxicosis. J Neurocrit Care. 2018; Sep. 11(2):129–33.

9. Kumar P, Panigrahi I, Sankhyan N, Ahuja C, Goyadi PK. Down syndrome with moyamoya disease: A case series. J Pediatr Neurosci. 2018; Apr-Jun. 13(2):201–4.

10. Kushima K, Satoh Y, Ban Y, Taniyama M, Ito K, Sugita K. Graves’ thyrotoxicosis and Moyamoya disease. Can J Neurol Sci. 1991; May. 18(2):140–2.

11. Russman AN, Katramados AM, Silver B, Mitsias PD. Moyamoya syndrome associated with Graves’ disease: a case report and review of the literature. J Stroke Cerebrovasc Dis. 2011; Nov. 20(6):528–36.

12. Menconi F, Marcocci C, Marinò M. Diagnosis and classification of Graves’ disease. Autoimmun Rev. 2014; Apr-May. 13(4-5):398–402.

13. Nakamura K, Yanaka K, Ihara S, Nose T. Multiple intracranial arterial stenoses around the circle of Willis in association with Graves’ disease: report of two cases. Neurosurgery. 2003; Nov. 53(5):1210–5.

14. Ngo SY, Chew HC. When the storm passes unnoticed--a case series of thyroid storm. Resuscitation. 2007; Jun. 73(3):485–90.

15. Nikiforov YE, Biddinger PW, Thompson LDR. Diagnostic Pathology and Molecular Genetics of the Thyroid. 2nd ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins;2012. p. X.
16. Noh BH, Cho S-W, Ahn SY. Simultaneous occurrence of diabetic ketoacidosis, thyroid storm, and multiple cerebral infarctions due to Moyamoya disease. J Pediatr Endocrinol Metab. 2016; Feb. 29(2):221–5.

17. Ohba S, Nakagawa T, Murakami H. Concurrent Graves’ disease and intracranial arterial stenosis/occlusion: special considerations regarding the state of thyroid function, etiology, and treatment. Neurosurg Rev. 2011; Jul. 34(3):297–304. discussion 304.

18. Ono Y, Ono S, Yasunaga H, Matsui H, Fushimi K, Tanaka Y. Factors associated with mortality of thyroid storm: analysis using a national inpatient database in Japan. Medicine (Baltimore). 2016; Feb. 95(7):e2848.
19. Peerless SJ. Risk factors of moyamoya disease in Canada and the USA. Clin Neurol Neurosurg. 1997; Oct. 99 Suppl 2:S45–8.

20. Ren S-C, Gao B-Q, Yang W-L, Feng W-X, Xu J, Li S-W, et al. Von Willebrand factor and coagulation factor VIII in Moyamoya disease associated with Graves’ disease: A case report. Exp Ther Med. 2016; Oct. 12(5):3195–200.

21. Sasaki T, Nogawa S, Amano T. Co-morbidity of moyamoya disease with Graves’ disease. Report of three cases and a review of the literature. Intern Med. 2006; 45(9):649–53.

22. Shen A, Ryu S, Lin S. Concurrent moyamoya disease and Graves’ thyrotoxicosis: case report and literature review. Acta Neurol Taiwan. 2006; Jun. 15(2):114–9.
Fig. 1.
Brain magnetic resonance imaging. (A) initial diffusion image, (B) aggravated diffusion image. Cerebral infarction territory at both frontal lobes was increased.
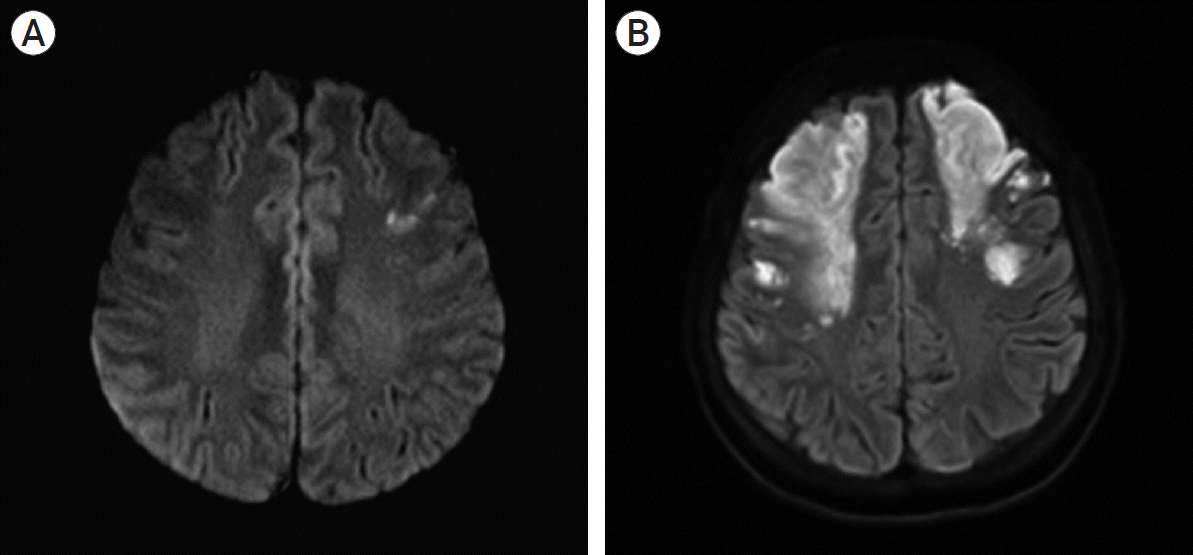
Fig. 2.
Cerebral angiography. (A) Right internal cerebral artery (ICA), (B) Left ICA, (C) Left vertebral artery. Steno-occlusion of bilateral distal ICA with moyamoya vessels.
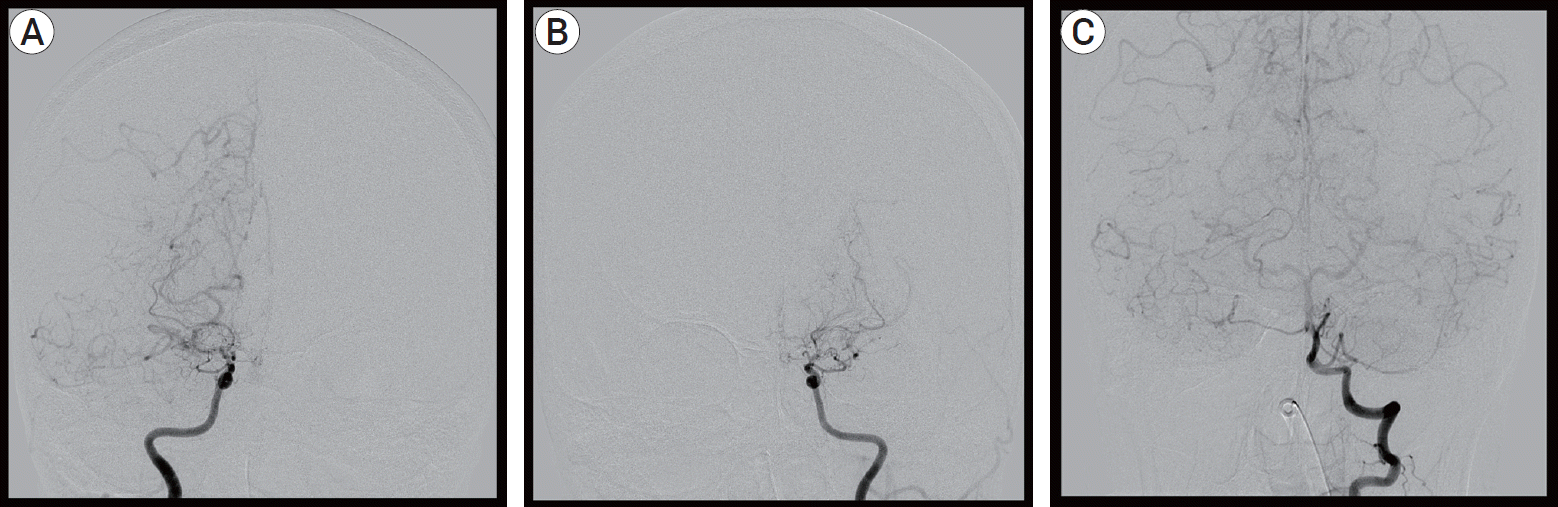
Table 1.
Summary of clinical data of all eight patients.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download