CASE PRESENTATION
A female in her late 40s, with history of tobacco abuse, was suffering a month of right eye transient ptosis. The patient was referred to the emergency room, where the neurological exam was unrevealing. Computed tomography (CT) angiography scan revealed no subarachnoid blood but showed multiple aneurysms on the left side, which included a lateral side wall anterior choroidal aneurysm measuring 5 mm, a posterior communicating (Pcomm) aneurysm measuring 3 mm, pouching from an infundibular dilatation, and a M1-2 segment 3 mm aneurysm. On the right, a 7-mm carotid terminus aneurysm was noted (
Fig. 1). The patient was admitted and underwent diagnostic subtraction angiography (DSA) which confirmed CT angiography findings, with highly unfavorable morphology of the left Pcomm and anterior choroidal aneurysms which were multilobulated with daughter sacs (
Fig. 1).
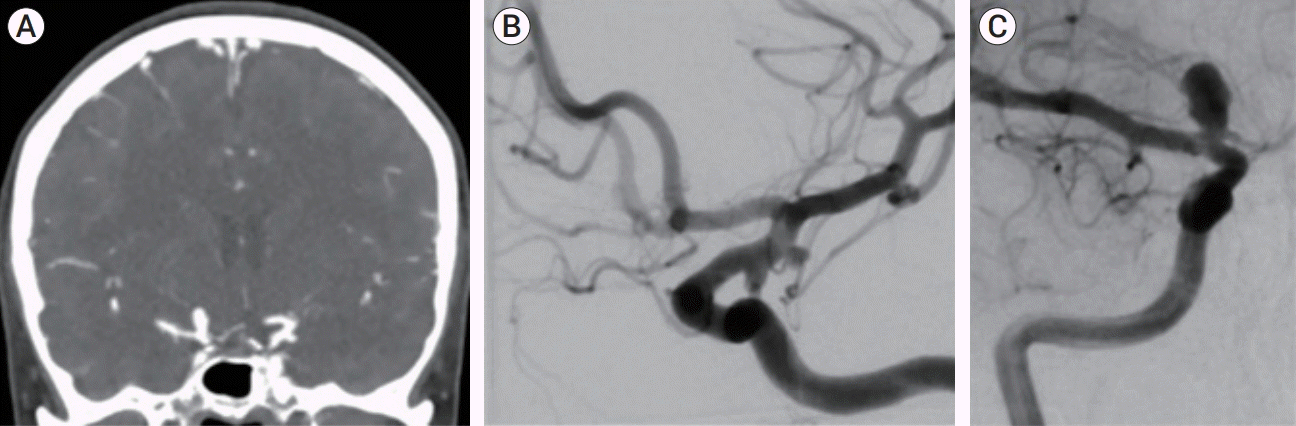 | Fig. 1.(A) CT angiography on admission showing multiple aneurysms. (B) DSA showing left lateral ICA wall and Pcomm aneurysms as well as M1-2 aneurysm. (C) Right carotid injection revealing the right carotid terminus aneurysm. CT, computed tomography; DSA, diagnostic subtraction angiography; ICA, internal carotid artery; Pcomm, posterior communicating. 
|
The patient was offered a graded treatment in the left Pcomm and anterior choroidal aneurysms first, due to their morphology, followed by a second session for the right internal carotid artery (ICA) aneurysm. The patient consented and under general anesthesia, underwent a therapeutic embolization. A CereBase
TM (J&J, New Brunswick, NJ, USA) long sheath catheter was placed in the subpetrosal segment of the left ICA. After 3D rotational angiography, cannulation of the left Pcomm aneurysm was completed using a Headway duo 167 Long
TM (MicroVention, Inc., Tustin, CA, USA) microcatheter over 0.014" Synchro2
TM wire (Stryker, Kalamazoo, MI, USA). A frame coil was then deployed using Target Nano
TM 1.5×2 coils (Stryker, Kalamazoo, MI, USA), partially occluding the daughter sac while preserving the flow through the left Pcomm (
Fig. 2).
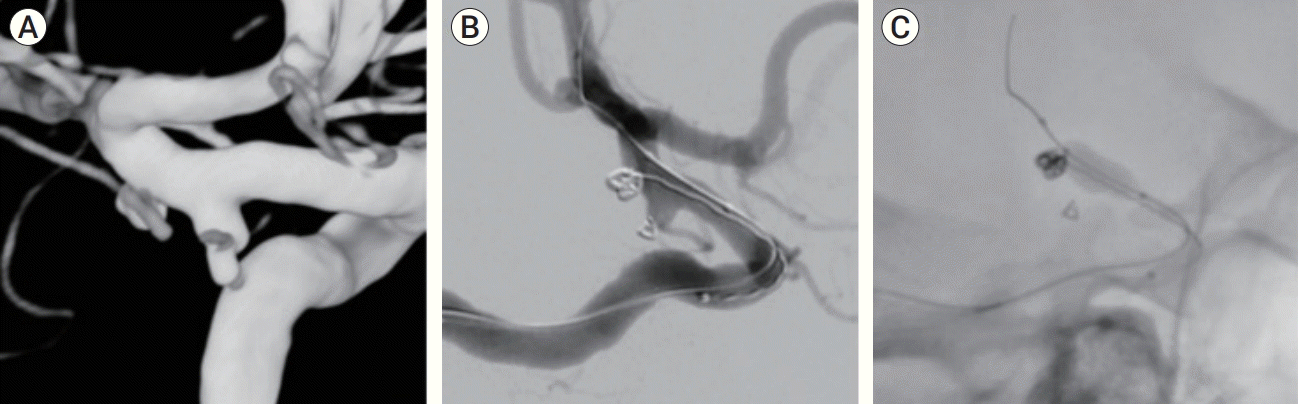 | Fig. 2.(A) Rotational 3D imaging showing the relationship between the Pcomm and the anterior choroidal aneurysms. (B) Pcomm aneurysm blebs secured, as coiling of the anterior choroidal aneurysm is taking place with frame coil buildup. (C) Balloon assisted coiling of the aneurysm. Pcomm, posterior communicating. 
|
Then, using a Headway17
TM (MicroVention, Inc., Tustin, CA, USA) microcatheter over the same wire, the anterior choroidal aneurysm was approached. A frame coil was deployed using a Target
TM 3D coil (Stryker, Kalamazoo, MI, USA), and using a balloon assisted technique, a Scepter XC
TM 4×11 mm balloon (MicroVention, Inc., Tustin, CA, USA) was placed over the aneurysm neck, resulting in a stable construct (
Fig. 2).
Then, Target NanoTM coil (Stryker, Kalamazoo, MI, USA), was introduced after microcatheter repositioning. Halfway through deployment, an unfavorable configuration of the nano coil was encountered. A decision was made to retrieve the coil, and it was pulled back into the microcatheter smoothly.
During this maneuver, the nano coil was re-deployed, and was brought into the microcatheter. Once the nano coil was within the microcatheter, having no contact with 3D frame coil, a swift migration of the frame coil construct was seen. The frame coil was then migrating backwards, into the microcatheter, protruding away from the aneurysm sac (
Fig. 3).
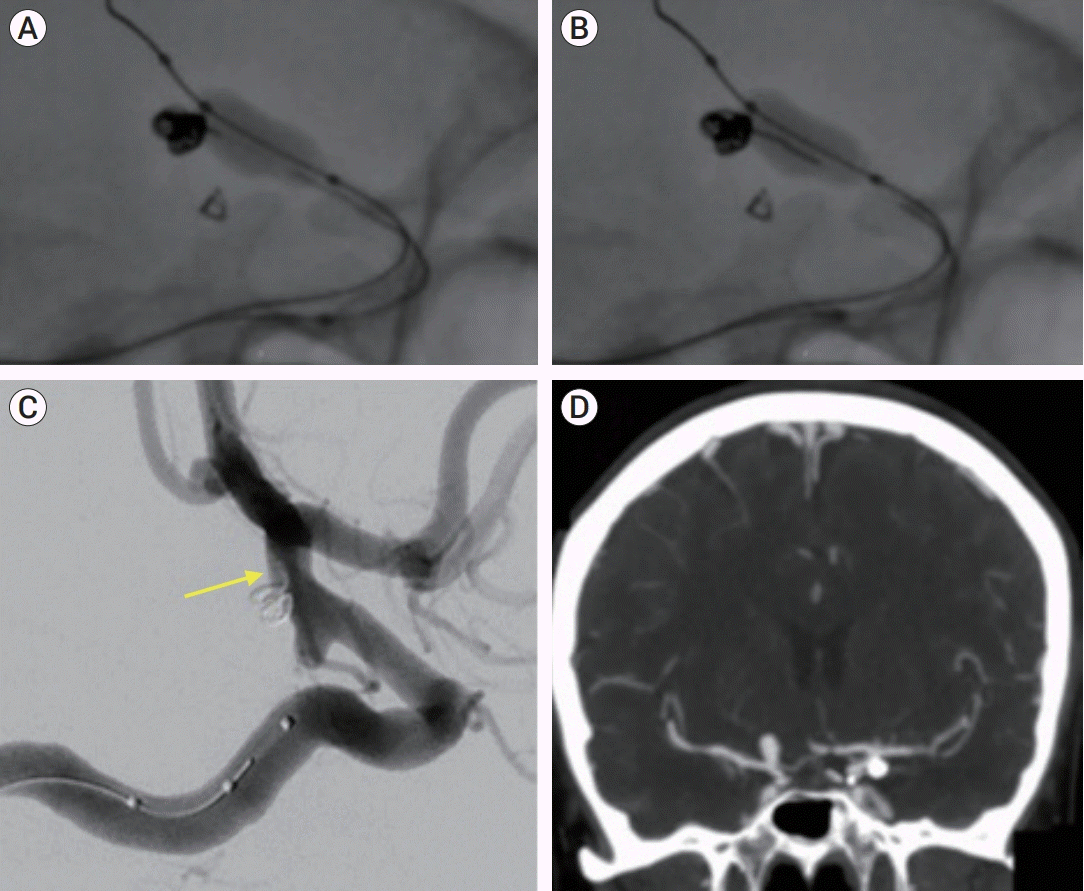 | Fig. 3.(A) Filling coil is retrieved into the microcatheter. Note a slight protrusion of the frame coil at the base of the aneurysm neck. (B) As the coil is further retrieved, simultaneous migration of the frame coil occurs into the catheter lumen. Notice the distance between the filling coil being retrieved and the frame coil, with a clear separation in between. (C) Using the balloon to stabilize further protrusion (see yellow arrow indicating coil protrusion in lateral ICA projection) and to redirect the frame coil free end back into aneurysm sac, a small loop is left adherent to the parent vessel lumen. (D) CT angiography showing final result. ICA, internal carotid artery; CT, computed tomography. 
|
With the aid of the nano coil, the frame coil was pushed through the catheter and re-positioned back into the aneurysm sac. Then again, the nano coil was retrieved, slowly down to the petrous ICA level. At this point the pulling was accelerated by the operator, and the frame coil again migrated away from the sac and into the microcatheter. A second attempt was made to re-position the frame coil but the microcatheter position was lost. At this stage, it was decided to inflate the balloon in order to stabilize the rest of the construct, and the microcatheter was fully removed leaving a small loop protruding adjacent to the ICA wall (
Fig. 3).
The frame coil remained stable, and all branches were seen patent. The procedure ended with both aneurysms secured (
Fig. 3), and the patient woke up with no neurological deficits, and was started on aspirin 100 mg tablets as a safety precaution. Patient was discharged on post-operative day #2 in her baseline status.
Go to :

DISCUSSION
Despite advances in technological and clinical utilization of endovascular techniques, catheters, coils and other devices may still behave unexpectedly during deployment. Coil migration, a phenomenon with an incidence of 0.5–6% [
1], may lead to a thrombogenic event due to occlusion of downstream vessels, or may shower emboli distally [
4]. Acute procedural migration is defined as coil migration that is detected during coiling procedure before arterial access closure, such as in this case.
Anatomical risk factors for coil migration include low aneurysm aspect ratios, small aneurysms, and communicating segment aneurysms [
5]. Technical risk factors include the use of small coils, undersizing coils relative to the maximal aneurysm size, manipulating first or last coil, and balloon deflation, which is related to inadvertent coil displacement [
2].
Coil retrieval may be regarded as cause for destabilizing previously deployed coils. However, relative movement of a coil, within the microcatheter with no contact with the migrating coil, “pulling” an already deployed coil, is not well reported and therefore warrants attention.
Importantly, Headway17
TM (MicroVention, Inc., Tustin, CA, USA) microcatheter internal diameter is 0.017 inches, while Target Nano
TM coil has an outer diameter of 0.010 inches, and all lines are continuously flushed during the procedure with heparinized saline solution. Thus, wire pulling backwards within the conduit, at such narrow lumens closely resembling in diameter, can potentially create a functional “venturi vacuum pump” [
3]. A venturi vacuum pump is based on the principle of the Conservation of Energy which states that, in a steady flow, the sum of all forms of mechanical energy in a fluid along a line is the same at all points. That is, the sum of the kinetic energy (reflected in the form of velocity) and potential energy (pressure) must remain constant. When a restriction is introduced in a microcatheter the velocity through that restriction must be greater than the velocity in the larger part of the conduit, since the same mass of fluid is flowing through a smaller cross-sectional area. Therefore, when an increase in the speed of the fluid occurs through the restricted section there is a proportionate decrease in pressure in order to maintain the same total mechanical energy. Further supporting this, is the fact that this phenomenon was initiated and accentuated by acceleration of coil retrieval (during the 2nd maneuver to retrieve the coil). Coil positioned within the microcatheter is narrowing it, and as it is pulled back, this “moving” constriction point within the catheter (at the interface coil-catheter), beyond threshold velocity, would generate immediate pressure decrease relative to non-constricted catheter tip, causing a “suction effect”. Therefore, with a rapid enough pull, the microcatheter distal tip may develop pressure drop sufficient to cause migration of the distal coil tip away from the aneurysm sac.
We therefore suggest that, since the microcatheter didn’t move during coil retrieval and since the frame coil was displaced directly during the same time the nano coil was retrieved down the microcatheter, a venturi-like vacuum effect can account for the frame coil “pulling” away from its initial position (
Fig. 4).
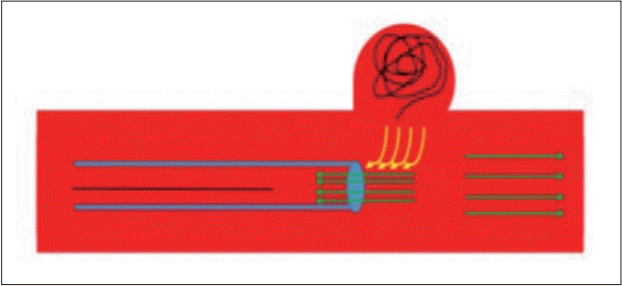 | Fig. 4.Venturi effect as a suggested mechanism promoting destabilization of the frame coil within the sac. By suction effect created locally by retrieving the coil within the microcatheter, creates vacuum at the relative narrowing site between catheter’s tip and the vessel lumen, which may disrupt frame coil initial position. 
|
Go to :

CONCLUSIONS
In conclusion, as a possible mechanism for coil migration “from afar”, we believe that raising the level of awareness to such an uncommon occurrence, is of clinical importance, and propose, that maintaining slow and gradual retrieval of a wire or a coil, even after the proximal tip is well away from the microcatheter tip, can potentially prevent this in future cases.
Go to :









 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download