Abstract
Purpose
The reconstruction of defects resulting from spinal surgery poses a challenge to plastic surgeons due to the curved contour and strong skin tension of the back. Implant and metal exposure can also increase the difficulty of covering such defects. This study presents our experiences of covering defects after spinal surgery using dorsal intercostal artery perforator (DICAP) flaps.
Methods
From November 2018 to August 2021, 14 patients with spinal soft tissue defects underwent DICAP flap reconstructive surgery at our department. The mean age of the patients was 54.3 years (range, 35–70 years). Age, sex, etiology, the dimensions of the defect and the flap, the site of the defect, surgical technique, and postoperative complications were recorded.
Results
All flaps survived, with no major complications such as total flap necrosis. Minor complications were observed in four cases. One patient developed an infection with erythematous changes and another developed partial flap necrosis. Two patients experienced wound dehiscence. These minor complications were all resolved with conservative treatment. No additional complications occurred during the follow-up period.
Spinal soft tissue defects resulting from spinal surgery complications are a major concern for reconstructive surgeons since they are difficult to reconstruct due to the low degree of vascularity in the area, strong skin tension, lack of tissue, and the curved contour of the back [1].
The standard procedure for addressing spinal soft tissue defects is first to administer conservative treatment including dressing and negative pressure treatment so that the defects reduce in size and can be surgically cleaned [2]. These defects are then covered with skin grafts, which are typically used to cover simple defects. However, when defects are located in a deep layer of tissue and neural structures or prosthetic materials are exposed, such treatment options are not effective. Traditionally, muscle or musculocutaneous flaps have been used to provide stable coverage for soft tissue defects [3]. The use of muscle flaps, however, can result in morbidity of the donor site [4].
Dorsal intercostal artery perforator (DICAP) flaps, which do not include muscle and cover well-vascularized tissue, have become a preferred option in reconstructive surgery [5,6]. DICAP flaps have the advantage of allowing large quantities of healthy vascular tissue to be transferred without damaging important underlying muscles. DICAP flaps have shown excellent results both functionally and cosmetically [7,8]. In this study, we described our experiences of using DICAP flaps to cover defects resulting from spinal surgery and its outcomes for treating spinal soft tissue defects.
Ethics statement: This study was approved by the Institutional Review Board of the Jeonbuk National University Hospital (No. 2022-05-032). All patients provided written informed consent to be included in the study and for their images to be used.
We performed a retrospective chart review of patients with spinal soft tissue defects resulting from spinal surgery between November 2018 and July 2021. For all 14 patients, data were collected on sex, age, etiology of their condition, the dimensions of the defect and the flap, and the site of the defect (Table 1). The defects were covered with DICAP flaps after the wounds were cleaned using debridement and irrigation.
All patients underwent surgery under general anesthesia in a prone position. For the first stage of the operation, unhealthy granulation tissue was carefully debrided and irrigated with saline solution to obtain a surgically clean wound. A hand-held Doppler (8 MHz, Bidop ES-100V3; Hadeco, Kawasaki, Japan) was used to mark the DICAP near the area of the defect. Next, we designed a boat-shaped DICAP flap using the tissue around the area of the defect. Careful dissection was then undertaken until the pedicle was revealed, and the DICAP flap was harvested. When elevating flap, we should take care not to dissect the pedicle and check pedicle with the hand-held Doppler if it is intact. In cases when there were large subcutaneous defects, the flaps were designed to be slightly larger than the size of the defect. Parts of the flaps were then deepithelized and used to fill the dead space.
The flap was then rotated 90° clockwise or counterclockwise and inset into the area of the defect, and a Hemovac drain was inserted. The flap was sutured and the donor site was primarily closed using the layer-by-layer technique.
The patients were instructed to avoid direct pressure on the reconstructed area for 2 weeks following the surgery. Conservative treatment was then undertaken and included antibiotics and dressing.
The patients with spinal soft tissue defects resulting from spinal surgery all received conservative treatment, including dressing with irrigation and antibiotics. After the wounds were surgically cleaned, all of the patients underwent reconstructive surgery using DICAP flaps.
All 14 patients were 11 males and three females, and the mean age was 55 years (range, 35–65 years). The average defect size was 46.25 cm2 (range, 15–150 cm2). The defects were located in the lumbar (10 patients, 71.4%), thoracic (three patients, 21.4%), and cervical (one patient, 7.1%) regions.
Postoperative flap complications were assessed for infection, wound dehiscence, partial necrosis, total necrosis, and the need for reoperation. The average length of hospital stay after wound closure was 12 days (range, 7–21 days). The mean follow-up period was 12 months (range, 1–25 months) (Table 1).
Stable wound healing was observed in all patients, with no instances of flap failure or necrosis after the completion of postoperative treatment. An infection developed in one case accompanied by erythematous change and discharge. Other complications included two cases of partial flap necrosis and one case of wound dehiscence. All complications were completely healed with conservative treatment. Aseptic dressing and intravenous antibiotic treatment were done to solve these minor complications. And necrotic tissues were debrided aseptically. With these treatments, infection subsided.
No indications of recurrence of infection were observed during the follow-up appointments of any of the patients, including through clinical signs, laboratory tests, and radiologic images. In all cases, the surgical site remained stable with durable coverage. No patients reported functional loss or discomfort at the donor site during the follow-up period.
A 51-year-old man visited our hospital due to operation site wound dehiscence after L4–L5 open lumbar microdiscectomy. Implant was used to fix vertebra exposed for 21 days after wound dehiscence. During the time, the patient received conservative treatment including dressing and intravenous antibiotics, and we planned to undertake an operation to cover the paraspinal defect after the wound cleared. The size of the defect was 8×4 cm2. DICAP flap surgery was therefore selected to cover the paraspinal defect.
The perforators were traced in the paraspinal area using a hand-held Doppler device. The flap was then dissected, rotated 90° clockwise, and inset into the area of the defect. The patient recovered without major complications, and 2 months after the surgery, the patient’s wound area had an acceptable cosmetic appearance (Fig. 1).
A 59-year-old man visited our hospital due to wound dehiscence after incision and drainage of a spinal abscess following T12–L2 screw fixation. The abscess that had formed around the operation site was incised for drainage to occur. However, the abscess recurred repeatedly due to the presence of the screw, and the patient was referred to our department for wound management. The implant was exposed for 30 days and we continued conservative treatment such as dressing and antibiotics during the time. The wound cleared, and we planned to undertake an operation to cover the paraspinal defect. We decided to perform DICAP flap surgery. The screws were removed, exposing dead space and the defect. The defect size was large, at about 10×4 cm2. The flap was designed, and part of the flap was deepithelized to fill the dead space at the metal removal site. The deepithelized flap was then buried and closed.
The patient recovered without major complications, and 6 months after the surgery, the patient’s wound area was stable (Fig. 2).
A 60-year-old man visited our hospital due to wound dehiscence after L2–L5 screw fixation. For 15 days, the implant was exposed and we continued conservative treatment. After that, the wound cleared, but an 8×4-cm2 defect remained. DICAP flap surgery was planned and performed.
After the surgery, we treated the site of the operation with dressing and intravenous antibiotics. However, partial necrosis was observed at the tip of the flap, and debridement of necrotic tissue was performed. The patient remained at the hospital for 2 weeks following the surgery with no complications. The patient was then discharged uneventfully, and 6 months after the surgery, the site of the operation was well-healed (Fig. 3).
After spinal surgery, there can be many complications such as wound necrosis and metal exposure that cause postoperative wound dehiscence and require coverage. In particular, patients with risk factors such as alcohol abuse, smoking, advanced age, steroid use, diabetes, obesity, and malnutrition are more likely to experience complications. Traditionally, complex soft tissue defects of the back have been reconstructed using muscle or musculocutaneous flaps, which are superior to random-pattern fasciocutaneous flaps [9]. Muscle flaps, including paraspinal muscle flaps, latissimus muscle flaps, trapezius muscle flaps, and pedicled flaps such as omental and superior gluteal flaps, have been widely used to cover defects. Reconstruction of soft tissue defects that occur after spinal surgery poses a challenge for reconstructive surgeons due to the low degree of vascularity in the area, strong skin tension, lack of tissue, the curved contour of the back, and the underlying diseases of patients. Moreover, when the implant is exposed, it is harder to cover areas of wound dehiscence, and defects can cause spinal instability, infection, and neurological deficits.
Accordingly, infection control before wound closure is important for reducing the risk of reconstructive failure. In the cases included in this study, infection was controlled using conservative treatment, and defects with spinal implants were covered using well-vascularized tissue and tension-free closure. In some studies, fasciocutaneous flaps are superior to muscle or musculocutaneous flaps in infection control. As such, fasciocutaneous flaps are appropriate for reconstruction and treatment of spinal surgery [10].
DICAP flaps present a new surgical option for treating paraspinal soft tissue defects, replacing muscle or musculocutaneous flaps as the preferred flaps in reconstructive surgical procedures [11]. DICAP flaps are vascularized using the intercostal perforator arteries. DICAP flaps are based on the perforators of the dorsal, dorsolateral, and lateral branches of the posterior intercostal artery in its vertebral, costal, intermuscular, and rectus segments, as well as the anterior intercostal branches of the internal mammary artery. DICAP flaps penetrate the intercostal muscles, and the perforators bifurcate into medial and lateral branches. Lateral branches are found on the midscapular line 5 to 8 cm from the midline. The medial branches are rooted within 5 cm of the spinous process and descend to the angle of the corresponding rib for 4 to 6 cm in a parallel manner (Fig. 4). As there are many connected perforators around the DICAP flap, it can be extended to capture adjacent perforasomes [12].
Some research has been conducted on the use of pedicled DICAP flaps. Infants with meningomyelocele, for example, have received DICAP flap surgery to cover large defects [13]. In addition, patients with latissimus dorsi donor-site wound dehiscence, dermatofibrosarcoma protuberans, and defects secondary to wound dehiscence after spinal surgery have, like in the present study, received DICAP flap surgery and have had good prognoses except for minor complications such as marginal necrosis [14].
We first debrided the unhealthy tissues and designed the DICAP flap containing the perforator of the dorsal intercostal artery, tracing it with a hand-held Doppler ultrasound device. We then dissected the flap until the pedicle was revealed and inset the DICAP flap into the defect area.
This flap preserves muscle function and minimizes morbidity at the donor site. In addition, it can be effectively used to treat more complicated defects such as soft tissue defects that include bone hardware exposure, providing well-vascularized flaps that are similar in color to the surrounding skin, satisfying patients both cosmetically and functionally.
This study has certain limitations, and further studies are needed that use a randomized, larger population and compare treatment options. Due to the relatively small number of cases, we could not identify the extent of complications or the limitations of the perforator flap. In addition, patients with underlying risk factors such as alcohol abuse, smoking, advanced age, steroid use, diabetes, obesity, and malnutrition are more likely to experience complications. In those patients, it is much more difficult to perform surgery and ensure appropriate wound hygiene. Some patients also had diabetes mellitus, which is known to affect the wound healing process. Since other risk factors were not controlled, this study had a limited ability to identify whether patients with diabetes mellitus were more likely to experience complications related to wound healing.
In cases where there is substantial dead space after spinal surgery, such as due to metal removal, the dead space can be filled with a deepithelized flap designed to be larger than the defect. The flap is then buried into the defect. A muscle flap, of course, is easier and more effective than a DICAP flap to fill dead space [15,16]. However, as deepithelized DICAP flaps have cosmetic advantages and lower donor-site morbidity, they can also be considered when there is substantial dead space following spinal surgery [17].
A paraspinal perforator flap enables the treatment of complex defects that either arise after spinal surgery or are associated with surgical site infections [18,19].
Spinal soft tissue defects and the surgical procedures used to treat them can have a major impact on patients’ quality of life. In our retrospective study of 14 patients, soft tissue defects around the spinal area were successfully treated using DICAP flaps with low donor-site morbidity.
There are many options for covering spinal area defects. However, covering defects after spinal surgery, especially when implants are exposed, requires the consideration of many problems. After DICAP flap surgery to cover spinal defects after spinal surgery, only minor complications were observed, and these complications healed well with conservative treatment. DICAP flaps are superior to muscle flaps since they have low donor-site morbidity and cosmetic advantages. DICAP flaps can be a useful option for covering spinal area defects.
References
1. DI Summa PG, Largo RD, Ismail T, et al. Reconstruction of spinal soft tissue defects with perforator flaps from the paraspinal region. In Vivo. 2019; 33:827–32.

2. Labler L, Keel M, Trentz O, Heinzelmann M. Wound conditioning by vacuum assisted closure (V.A.C.) in postoperative infections after dorsal spine surgery. Eur Spine J. 2006; 15:1388–96.

3. Mericli AF, Mirzabeigi MN, Moore JH Jr, Fox JW 4th, Copit SE, Tuma GA. Reconstruction of complex posterior cervical spine wounds using the paraspinous muscle flap. Plast Reconstr Surg. 2011; 128:148–53.

4. May JW Jr, Rohrich RJ. Foot reconstruction using free microvascular muscle flaps with skin grafts. Clin Plast Surg. 1986; 13:681–9.

5. de Weerd L, Weum S. The sensate medial dorsal intercostal artery perforator flap for closure of cervicothoracic midline defects after spinal surgery: an anatomic study and case reports. Ann Plast Surg. 2009; 63:418–21.
6. Weum S, de Weerd L. The sensate medial dorsal intercostal artery perforator flap as an option for treatment of dorsal cervicothoracic midline defects. Plast Reconstr Surg. 2010; 126:1122–4.

7. Lazzeri D, Huemer GM, Nicoli F, et al. Indications, outcomes, and complications of pedicled propeller perforator flaps for upper body defects: a systematic review. Arch Plast Surg. 2013; 40:44–50.

8. Oh TS, Hallock G, Hong JP. Freestyle propeller flaps to reconstruct defects of the posterior trunk: a simple approach to a difficult problem. Ann Plast Surg. 2012; 68:79–82.

9. Garvey PB, Rhines LD, Dong W, Chang DW. Immediate soft-tissue reconstruction for complex defects of the spine following surgery for spinal neoplasms. Plast Reconstr Surg. 2010; 125:1460–6.

10. Kovar A, Colakoglu S, Iorio ML. Choosing between muscle and fasciocutaneous free flap reconstruction in the treatment of lower extremity osteomyelitis: available evidence for a function-specific approach. J Reconstr Microsurg. 2020; 36:197–203.

11. de Weerd L, Solberg TK, Weum S. Closure of complex posterior midline defects after spinal surgery with sensate midline-based perforator flaps and the long-term results. Spine (Phila Pa 1976). 2015; 40:E1233–8.

12. Hamdi M, Van Landuyt K, de Frene B, Roche N, Blondeel P, Monstrey S. The versatility of the inter-costal artery perforator (ICAP) flaps. J Plast Reconstr Aesthet Surg. 2006; 59:644–52.

13. Kocak OF, Demir CY. An ideal flap alternative for closure of myelomeningocele defects: dorsal intercostal artery perforator flap. J Craniofac Surg. 2016; 27:1951–5.
14. Prasad V, Morris SF. Propeller DICAP flap for a large defect on the back-case report and review of the literature. Microsurgery. 2012; 32:617–21.

15. Mathes DW, Thornton JF, Rohrich RJ. Management of posterior trunk defects. Plast Reconstr Surg. 2006; 118:73e–83e.

16. Saint-Cyr M, Nikolis A, Moumdjian R, et al. Paraspinous muscle flaps for the treatment and prevention of cerebrospinal fluid fistulas in neurosurgery. Spine (Phila Pa 1976). 2003; 28:E86–92.

17. Datli A, Suh H, Kim YC, Choi DH, Hong JP. Free-style deepithelialized propeller flaps: an ideal local flap to obliterate wounds with dead space. Plast Reconstr Surg Glob Open. 2017; 5:e1249.
Fig. 1.
A dorsal intercostal artery perforator (DICAP) flap in a 51-year-old man. (A) DICAP flap design using a hand-held Doppler device. (B) After dissection including thoracolumbar fascia, the flap was elevated. (C) The flap was rotated 90° clockwise. (D) Results at 2 months postoperative.
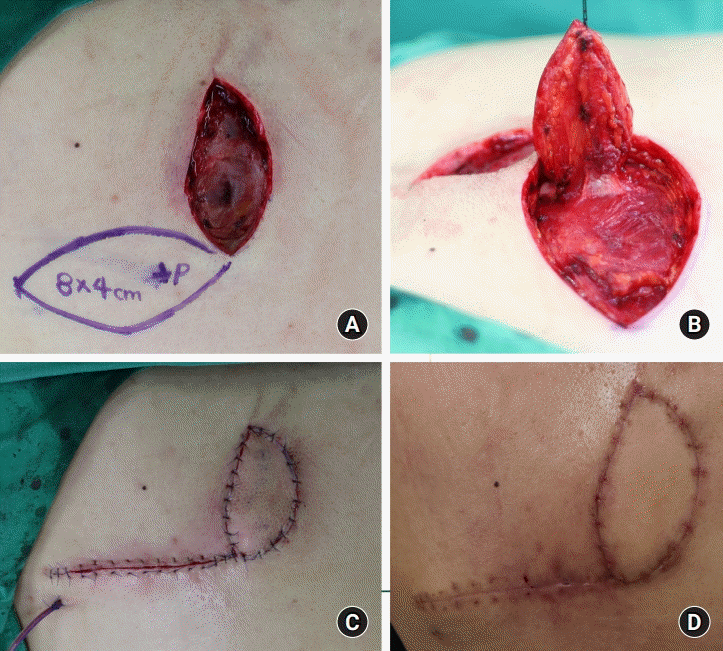
Fig. 2.
A dorsal intercostal artery perforator (DICAP) flap in a 59-year-old man. (A) DICAP flap design using a hand-held Doppler device. (B) After dissection including thoracolumbar fascia, the flap was elevated. (C) The flap was de-epithelized. (D) Immediate postoperative images.
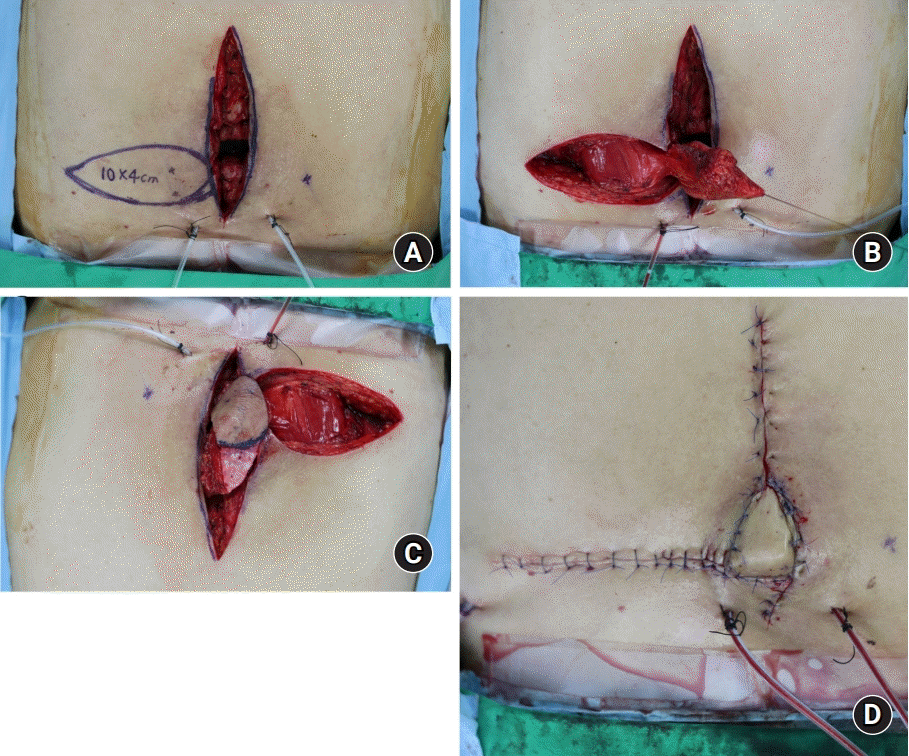
Fig. 3.
A dorsal intercostal artery perforator (DICAP) flap in a 60-year-old man. (A) DICAP flap design using a hand-held Doppler device. (B) After dissection including thoracolumbar fascia, the flap was elevated (C) The flap was rotated 90° clockwise. (D) Partial necrosis was observed at the tip of the flap.
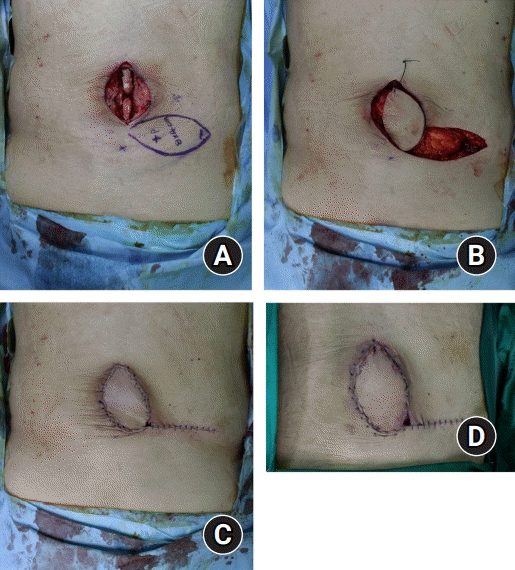
Fig. 4.
Schematic illustration of the cross-section of an intercostal space. DICAP, dorsal intercostal artery perforator; LICAP, lateral intercostal artery perforator; V, vertebra; A, aorta; PICA, posterior intercostal artery.
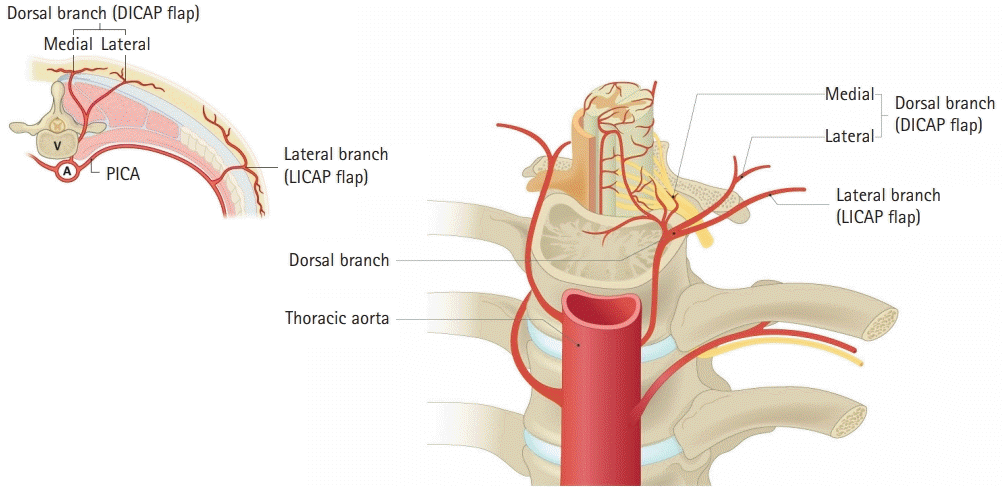
Table 1.
Clinical analysis of patients




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download