Abstract
Purpose
Local corticosteroid injections are routinely used as first-line treatment for trigger finger. The accurate delivery of steroids into the tendon sheath is important for the effectiveness of the treatment and the prevention of complications. This study aimed to introduce our steroid injection technique for trigger finger, which uses tendon excursion of the flexor tendon, and evaluate the clinical outcomes in patients who were treated with this technique.
Methods
A total of 171 patients with trigger finger who were treated with steroid injections were retrospectively reviewed. The efficacy of injection and complications were investigated. The efficacy was evaluated as“good,” “fair,” or “poor.” The results were analyzed according to the type of finger and the Quinnell grading system.
Results
The total efficacy was 83.6% (good/fair, 143 digits; poor, 28 digits). The treatment success rate for Quinnell grade IV was 43.8% (7 of 16). This rate was significantly lower than those of Quinnell grades II and III, which were 88.9% (88 of 99) and 87.5% (49 of 56), respectively (II vs. IV, p=0.004; III vs. IV, p=0.010). The success rate for the four fingers excluding the thumb was significantly higher than that of the thumb (88.2% vs. 75.4%, p=0.048).
Trigger finger, which is also known as stenosing tenosynovitis, is caused by the thickening of the first annular (A1) pulley surrounding the flexor tendon of the finger. It is the most common hand disease that causes pain and dysfunction of the hand [1]. Trigger finger can be nonsurgically treated using splints, nonsteroidal anti-inflammatory drugs, or conservative treatment by injecting steroids into the aponeurosis. Surgical methods such as percutaneous incision or invasive A1 pulley incision are performed when conservative methods are ineffective [1-3].
Local steroid injection in the trigger finger, which was first reported in 1953 by Howard et al. [4], is a convenient technique and produces excellent results (therapeutic efficiency of 60%–93%) [5-7]. It is important to accurately inject the steroid into the flexor tendon sheath to increase the therapeutic effect of steroids and reduce side effects such as tendon rupture, skin atrophy, and discoloration, which can occur because of local injection [8,9]. However, there is only a 36% to 49% probability that the drug will be accurately injected into the flexor tendon sheath during actual steroid injection [10,11], and only a few studies have reported the steroid injection technique and its clinical results in trigger finger.
This study aimed to introduce our steroid injection technique for trigger finger, which uses tendon excursion of the flexor tendon, and to evaluate the clinical outcomes in patients who received this technique as the primary treatment. Our hypothesis was that the technique simultaneously provided satisfactory finger function restoration and low complication rates.
Ethics statements: This study was performed in accordance with the Declaration of Helsinki. The design and protocol of this study were reviewed and approved by the Institutional Review Board (IRB) of Inha University Hospital (No. INHAUH 2019-09-008). Verbal informed consent was obtained from all participants prior to the study. Written informed consent was waived by the IRB because of its retrospective nature via medical records.
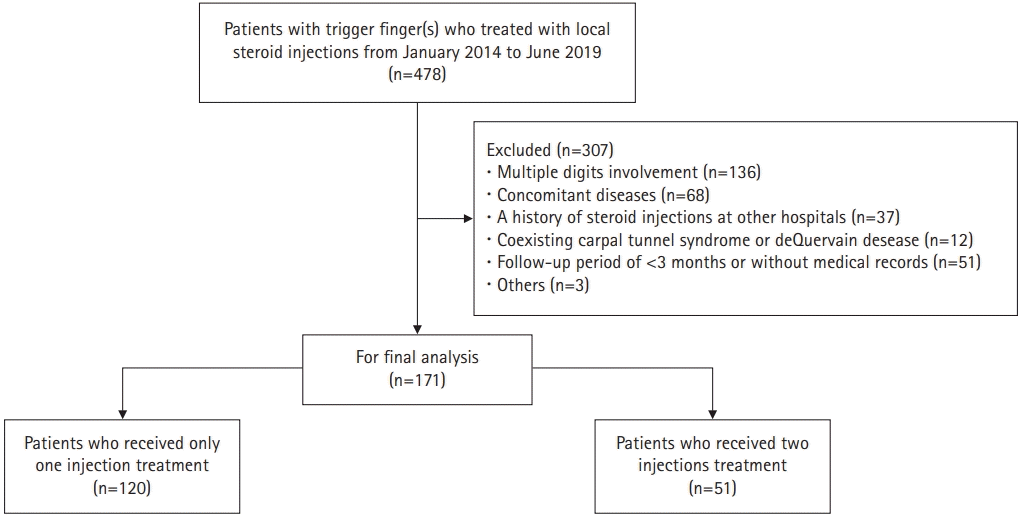
From January 2014 to June 2019, 478 patients with trigger finger who were treated with local steroid injections at our institution were retrospectively reviewed. The study subjects included patients with idiopathic trigger finger who were followed up for at least three months after steroid injection treatment. The diagnosis of idiopathic trigger finger was made on the basis of clinical assessments by an orthopedic hand surgeon at the first outpatient visit. The exclusion criteria included congenital trigger finger, diseases that involve the joints as rheumatoid arthritis or other connective tissue diseases, concomitant diseases that affect prognosis such as diabetes mellitus and/or chronic kidney disease, and coexisting symptomatic carpal tunnel syndrome or de Quervain’s disease. Patients who had a history of steroid injections at other hospitals or had undergone surgical treatment, those who were followed up for <3 months, or those with multiple digit involvement were also excluded from this study.
All patients were injected by an experienced orthopedic hand surgeon (T.J.L.) on an outpatient basis by using the following method (Fig. 1, Supplementary Video 1). With the patient lying in the supine position, the nodule of the flexor tendon was palpated on the A1 pulley, and the patient’s palm was extensively sterilized with alcohol (Fig. 1A). With the affected finger in a flexed state, a 25-gauge needle was inserted deep into the flexor tendon at a 45° angle from the proximal to the distal part of the A1 pulley (Fig. 1B). After bending and extending the finger, it was confirmed that the needle moved with the movement of the finger (Fig. 1C). After repeating the flexion and extension movements of the finger until the needle no longer moved, a mixture consisting of 0.5 mL of 40 mg/mL triamcinolone acetonide (Triam Inj.; Shin Poong Pharm Co., Ltd., Seoul, Korea) and 0.5 mL of 1% lidocaine (Huons Lidocaine HCl 1%; Huons Co., Ltd., Seongnam, Korea) was injected. Intrasheath injection was confirmed by feeling a fluid thrill without resistance by palpating the affected finger during injection. After injection, the fluid-filled synovium along the flexor tendon was visually observed (Fig. 1D). After the injection treatment, movement of the fingers was allowed immediately. Immobilization of the splint was not performed. After the first steroid injection, follow-up was performed for at least 3 months. If symptoms did not improve, the injection was readministered with an interval of at least 6 weeks, and the number of injections per digit did not exceed two injections. After two injections without benefit, patients were encouraged to pursue surgical management (open A1 pulley release).
The Quinnell grading system [12] was used to assess the clinical severity of the trigger finger. On the basis of this classification, the patients were rated as follows at the first outpatient visit: 0, normal movement; I, uneven movement without locking; II, actively correctable intermittent locking; III, passively correctable intermittent locking; and IV, locked digit (Table 1) [12]. The treatment results of steroid injection, changes in pain and symptoms, and presence or absence of newly developed side effects were assessed during follow-up. The evaluation of the treatment effect was classified into “good,” “fair,” and “poor” according to the status during the follow-up period. A case was graded as “good” when pain and trigger phenomena completely disappeared and did not recur in the follow-up period. A case was graded as “fair” if some symptoms remained but caused no discomfort in daily life. Patients with “good” and “fair” grades were considered satisfied with their results. A case was graded as “poor” if the symptoms did not disappear even after two injections or temporarily improved but recurred to the state before the injection and if the surgical treatment was performed within the final follow-up period.
The data are presented as mean±standard deviation or as numbers and percentages. The treatment results of the thumb and other fingers were compared using Pearson chi-square test. Post hoc tests were used following Pearson chi-square test to compare the success rates according to the Quinnell grade. The analysis was conducted using IBM SPSS Statistics ver. 25.0 for Windows (IBM Corp., Armonk, NY, USA). Statistical significance was set at p<0.050.
Among the 478 patients, 171 digits (171 patients) were enrolled in this study after applying the inclusion and exclusion criteria (Fig. 2). Table 2 shows a summary of the patient demographics. There were 51 males (29.8%) and 120 females (70.2%), and the mean age was 56.1±12.0 years (range, 17–83 years). The mean follow-up period after the first injection was 17.6±14.9 months (range, 3–54 months). The most commonly affected digit was the thumb (69 digits [40.4%]), followed by the index finger (28 digits [16.4%]), middle finger (58 digits [33.9%]), ring finger (26 digits [15.2%]), and little finger (4 cases [2.3%]) (Table 2). According to the Quinnell grading system, there were 99 digits of grade II (57.9%), 56 digits of grade III (32.7%), and 16 digits of grade IV (9.4%). Patients with grades 0 or I that were not considered for injections were not included in this study (Table 1).
The total efficacy was 83.6% (good/fair, 143 digits; poor, 28 digits) (Table 3). Among 171 digits, 120 digits (70.2%) showed complete recovery after the first steroid injection, and no recurrence of symptoms was observed within the follow-up period. Fifty-one digits (29.8%) showed no improvements in symptoms or showed recurrence of symptoms within the follow-up period and received the second injection treatment after 6 weeks of follow-up. By contrast, symptoms were relieved in 23 digits (13.5%). Among the 28 digits (16.4%) that did not improve their symptoms even after two steroid injections, 25 digits (15.2%) underwent surgical treatment (open A1 pulley release), and three patients withdrew from the follow-up.
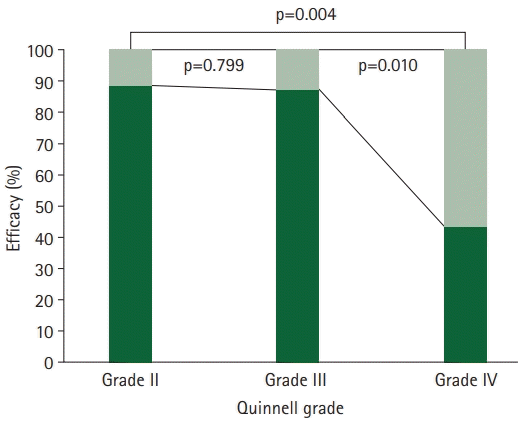
The treatment success rate for Quinnell grade IV was 43.8% (7 of 16), which was significantly lower than those of Quinnell grades II and III, which were 88.9% (88 of 99) and 87.5% (49 of 56), respectively (II vs. IV, p=0.004; III vs. IV, p=0.010) (Fig. 3).
In the case of the thumb, 53 out of 69 cases (76.8%) showed improvements in symptoms, and the other four fingers (excluding the thumb) showed improvements in symptoms in 90 out of 102 cases (88.2%). For the other fingers (excluding the thumb), the success rate was significantly higher than that of the thumb (p=0.048) (Table 3).
During the follow-up period, one digit (0.6%) with subcutaneous tissue atrophy and two digits (1.2%) with skin depigmentation were observed (Fig. 4). Superficial infection occurred in two digits (1.2%), which were treated with oral antibiotics. There were no cases of tendon rupture, nerve damage, or blood vessel damage.
Trigger finger, which was first described by Notta [13] in 1850, is a stenosing tenosynovitis of the flexor tendon that causes pain and dysfunction of the hand [1]. Ever since Howard et al. [4] first used hydrocortisone injection as a treatment for trigger finger, therapeutic results of 60%–93% have been reported, and local steroid injection is known as the primary treatment method for trigger finger [5-7]. In the present study, the efficacy of our steroid injection technique was investigated. Our injection technique uses tendon excursion during injec-tion to accurately deliver steroids into the flexor tendon sheath. It showed excellent results and low complication rates.
To increase the therapeutic effect of topical steroid injection, it is important to accurately inject the steroid into the flexor tendon sheath [8]. However, there is a low probability that the steroid will be accurately injected into the flexor tendon sheath during blind steroid injection. In a study by Kamhin et al. [10], the distribution of methylene blue was visually confirmed during surgical treatment after injecting methylene blue solution into the flexor tendon sheath of 77 patients scheduled for surgical treatment of trigger finger. Only 38 patients (49.4%) reported that methylene blue was injected into the flexor tendon sheath. In a study by Fowler et al. [11], the distribution of iopamidol contrast dye was examined by live fluoroscopy in 25 patients; only nine patients (36.0%) reported that the dye was injected into the flexor tendon sheath. To accurately inject steroids into the tendon sheath during blind injections, Freiberg et al. [14] inserted the needle deep into the flexion tendon from the proximal part of the A1 pulley, extended and flexed the finger to observe that the needle moved together, and then withdrew the needle gradually until the needle did not move with the finger. It was suggested that the drug should be injected at the position where the needle did not move while it was being pulled out. This is similar to our injection technique, which uses tendon excursion, but it is different in that the needle is pulled out of the flexor tendon by repeatedly performing extension and flexion movements of the finger instead of withdrawing the needle.
During injection, if the finger is well flexed and extended, the needle can come out of the flexor tendon well. In other words, with proper tendon excursion during injection, the tip of the needle can be positioned into the flexor tendon sheath (Fig. 5). In the present study, the efficacy of steroid injection in the other fingers (except the thumb) was 88.2%, which was higher than that seen in the thumb at 75.4% (p=0.048). We thought that the reason why the treatment results of the thumb were not as good as those of other fingers could be the difference in the accuracy of the injection location between the thumb and other fingers. The distance of tendon excursion is generally 3.0 to 3.4 cm for the flexor digitorum profundus (FDP) and 2.8 cm for the flexor pollicis longus (FPL) in the neutral state of the wrist [15]. Because the distance of tendon excursion of FDP is longer than that of FPL, there may be differences in the accuracy of the injection location. We believe that these differences could affect the difference in treatment outcomes between the thumb and other fingers.
In the trigger finger, as the A1 pulley surrounding the flexor tendon thickens, the tunnel for tendon excursion narrows, and the tendon excursion of the flexor tendon becomes limited [1]. Quinnell [12] classified the stages of the trigger finger by its symptoms. In fingers of Quinnell grade IV, there were limitations in our injection technique due to fixed tendon excursion because such fingers are fixed in a bent or nonflexed state. In this study, 16 patients (9.4%) had grade IV disease, and the treatment rate was 43.8% (7 of 16), which was significantly lower than those of Quinell grade II (88.9%, 88 of 99) and grade III (87.5%, 49 of 56). This difference in the treatment rate may be caused not only by the difference in the degree of initial symptoms but also by the possibility that inaccurate injection occurred because of the limitation in the tendon excursion distance due to the restriction of the extension movement during injection.
Although local steroid injections are effective for treating various musculoskeletal disorders, complications may arise. The potential complications of local steroid injection include infection, skin depigmentation, tendon rupture, and fat atrophy [8]. The injection site is crucial when administering a steroid injection (Fig. 6). If the steroid injection is intratendinous, it is highly likely to damage the tendon tissue and lead to necrosis of the collagen bundle of the tendon, which results in tendon rupture [16,17]. There have been a few reports of rupture of the flexor digitorum tendon after steroid injection for trigger finger [18]. Several studies have been reported that extrasheath injection is as effective as intrasheath injection [19,20]. However, extrasheath injection has the potential to cause skin depigmentation and/or fat atrophy because the steroid spreads into subcutaneous tissue. It has been reported that fat atrophy can cause 1% depigmentation in 5% of patients after local steroid injection [21]. In the present study, the results showed a low incidence of complications; only one digit (0.6%) had subcutaneous tissue atrophy, and only two digits (1.2%) had skin depigmentation. We believe that the reason for the relatively low incidence of complications is that our technique induces intrasheath injection as much as possible. In addition, the fact that the injection per digit was not performed more than twice and that the second injection was performed at an interval of more than 6 weeks may have reduced complications.
Ultrasound-guided injection is a widely used technique for intrasheath injection and has been reported to have excellent treatment rates of 91.4% to 93.4% [22,23]. However, it requires a lot of time and is expensive compared with blind injection techniques. On the other hand, our injection technique is simple, quick, inexpensive, and easy to follow even by unskilled operators.
This study had several limitations. First, this was a retrospective study, and there was no control group that did not use our technique. Therefore, it was difficult to analyze the therapeutic effect of injections using our technique. Randomized controlled trials may be needed to provide a higher level of evidence. Second, our technique is to inject into the tendon sheath, which first requires intratendon needling. Although steroids are not injected directly into the tendon, it has the potential to cause microtrauma to the tendon. Finally, the confirmation of whether steroids were accurately delivered into the flexor tendon sheath was limited. Intrasheath injection was judged via the naked eye and by palpation of thrills. It may be necessary to accurately check whether the steroid is injected into the intrasheath by using ultrasound. Despite these limitations, our findings are meaningful because they provide evidence of the clinical effects of our technique and are valuable as a report that introduces our injection technique for trigger finger.
The steroid injection technique using tendon excursion of the trigger finger showed excellent results and low rate of complications. In particular, second to fourth fingers with longer tendon excursions showed more effective results than the thumb. Fingers of Quinnell grade IV with poor tendon excursion showed lower treatment results.
Supplementary materials
Supplementary Video 1 can be found via https://doi.org/10.12790/ahm.21.0134.
References
1. Ryzewicz M, Wolf JM. Trigger digits: principles, management, and complications. J Hand Surg Am. 2006; 31:135–46.

2. Huisstede BM, Hoogvliet P, Coert JH, Fridén J; European HANDGUIDE Group. Multidisciplinary consensus guideline for managing trigger finger: results from the European HANDGUIDE Study. Phys Ther. 2014; 94:1421–33.

3. Lunsford D, Valdes K, Hengy S. Conservative management of trigger finger: a systematic review. J Hand Ther. 2019; 32:212–21.

4. Howard LD Jr, Pratt DR, Bunnell S. The use of compound F (hydrocortone) in operative and non-operative conditions of the hand. J Bone Joint Surg Am. 1953; 35-A:994–1002.

5. Anderson B, Kaye S. Treatment of flexor tenosynovitis of the hand (‘trigger finger’) with corticosteroids: a prospective study of the response to local injection. Arch Intern Med. 1991; 151:153–6.

6. Benson LS, Ptaszek AJ. Injection versus surgery in the treatment of trigger finger. J Hand Surg Am. 1997; 22:138–44.

7. Murphy D, Failla JM, Koniuch MP. Steroid versus placebo injection for trigger finger. J Hand Surg Am. 1995; 20:628–31.

8. Nichols AW. Complications associated with the use of corticosteroids in the treatment of athletic injuries. Clin J Sport Med. 2005; 15:370–5.

9. Akhtar S, Burke FD. Study to outline the efficacy and illustrate techniques for steroid injection for trigger finger and thumb. Postgrad Med J. 2006; 82:763–6.

11. Fowler JR, Ogrich L, Evangelista P, Schaffer AA. Assessing injection techniques in the treatment of trigger finger. Mod Plast Surg. 2012; 2:83–6.
12. Amiri Aref H, Fatemeh S, Mohammad Hosein K. Comparison between corticosteroid injection and surgery in the treatment of trigger finger. J Transl Int Med. 2015; 2:132–5.

13. Notta A. Recherches sur une affection particuliere des gaines tendineuses de la main, caracterisee par le developement de une nodosite sur la trajet des tendons flechisseurs des doigts et par l'empechment de leurs mouvements. Arch Gen Med. 1850; 24:142–61.
14. Freiberg A, Mulholland RS, Levine R. Nonoperative treatment of trigger fingers and thumbs. J Hand Surg Am. 1989; 14:553–8.

15. Franko OI, Winters TM, Tirrell TF, Hentzen ER, Lieber RL. Moment arms of the human digital flexors. J Biomech. 2011; 44:1987–90.

16. Balasubramaniam P, Prathap K. The effect of injection of hydrocortisone into rabbit calcaneal tendons. J Bone Joint Surg Br. 1972; 54:729–34.

17. Lin YC, Shieh SJ. Extensor-pollicis-longus or -brevis tendon rupture after corticosteroid injection. Formos J Surg. 2016; 49:15–9.

18. Fitzgerald BT, Hofmeister EP, Fan RA, Thompson MA. Delayed flexor digitorum superficialis and profundus ruptures in a trigger finger after a steroid injection: a case report. J Hand Surg Am. 2005; 30:479–82.

19. Shinomiya R, Sunagawa T, Nakashima Y, Yoshizuka M, Adachi N. Impact of corticosteroid injection site on the treatment success rate of trigger finger: a prospective study comparing ultrasound-guided true intra-sheath and true extra-sheath injections. Ultrasound Med Biol. 2016; 42:2203–8.

20. Mardani-Kivi M, Karimi-Mobarakeh M, Babaei Jandaghi A, Keyhani S, Saheb-Ekhtiari K, Hashemi-Motlagh K. Intra-sheath versus extra-sheath ultrasound guided corticosteroid injection for trigger finger: a triple blinded randomized clinical trial. Phys Sportsmed. 2018; 46:93–7.

21. Brinks A, Koes BW, Volkers AC, Verhaar JA, Bierma-Zeinstra SM. Adverse effects of extra-articular corticosteroid injections: a systematic review. BMC Musculoskelet Disord. 2010; 11:206.

22. Godey SK, Bhatti WA, Watson JS, Bayat A. A technique for accurate and safe injection of steroid in trigger digits using ultrasound guidance. Acta Orthop Belg. 2006; 72:633–4.
Fig. 1.
Photograph demonstrating the injection technique using tendon excursion in trigger finger. (A) Palpitate the metacarpal neck. (B) With the affected finger in a flexed position, insert a 26-gauge needle aiming at 45°. (C) Ask the patient to flex and extend the finger to ascertain that the needle tip is not in the tendon. (D) Inject and feel the fluid thrill.
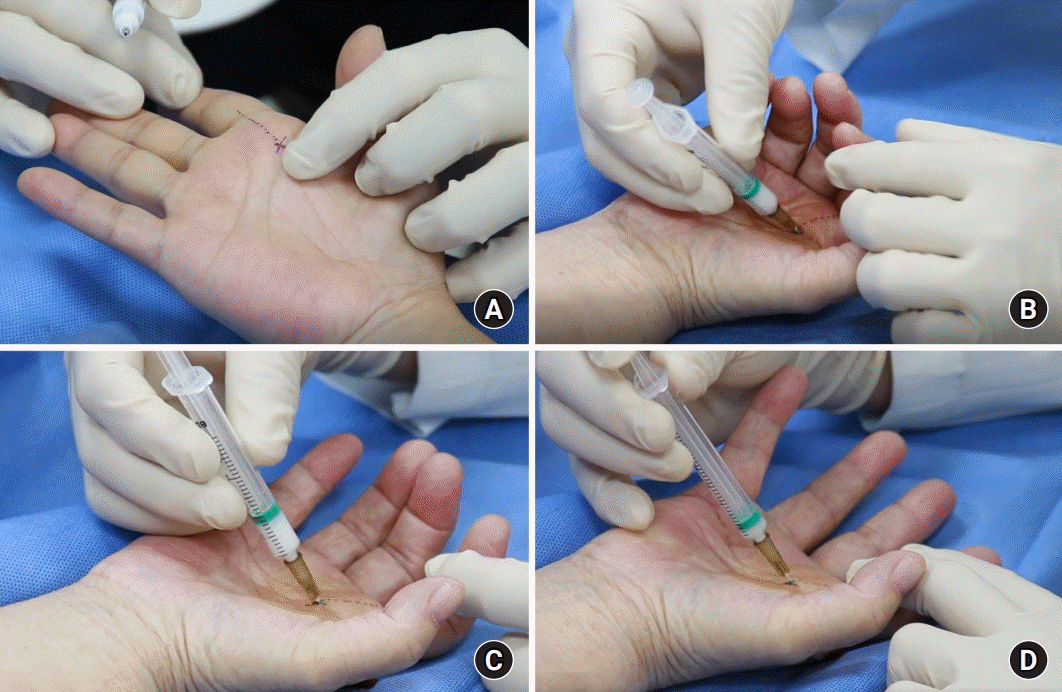
Fig. 4.
A 56-year-old female patient with right trigger thumb developed complications after the second steroid injection. The photograph shows skin depigmentation and fat atrophy of the right thumb.
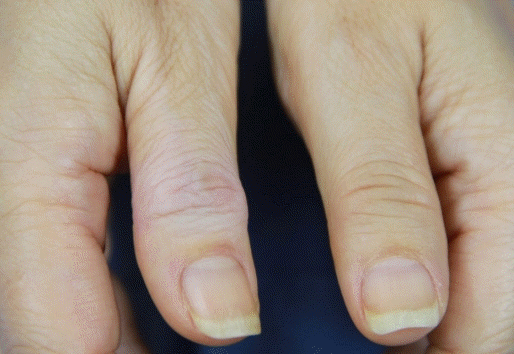
Fig. 5.
Diagram showing the needle tip position during tendon excursion. (A) Insertion of the needle tip up to the tendon in the bevel-up state. (B) Rotation of the needle tip 180° to bevel down. (C) View of the needle entering into the flexor sheath after tendon excursion but not passing through the tendon substance.
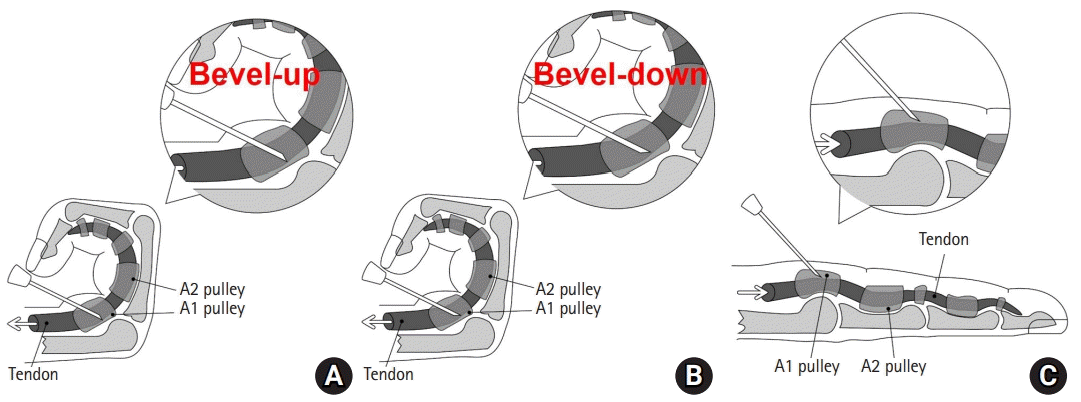
Fig. 6.
Cross-sectional view showing three possible positions of the needle tip in the A1 pulley. (A) Intratendinous injection, (B) intrasheath injection, and (C) extrasheath injection.
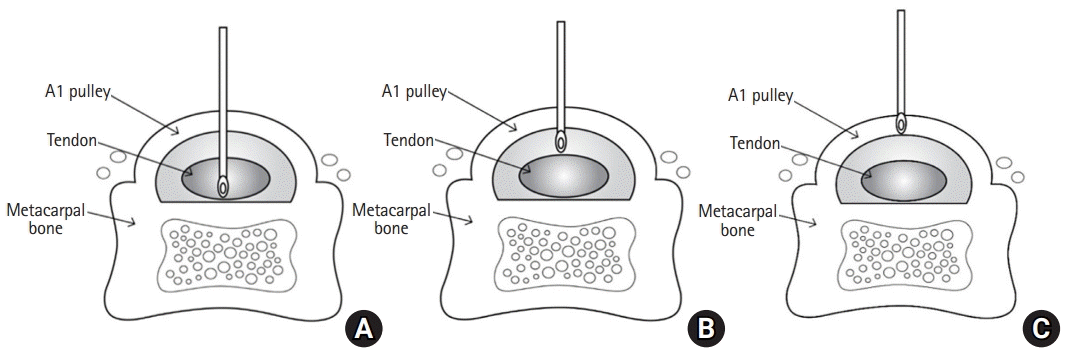
Table 1.
Quinnell grading of trigger finger [12]
Values are presented as number (%).
Modified from Amiri Aref et al. [12] according to the Creative Commons License.
Table 2.
Patients' demographics
Table 3.
Comparison of clinical outcomes between the thumb and other fingers
| Variable |
Affected digit |
p-valuea) | ||||||
|---|---|---|---|---|---|---|---|---|
| All (n=171) | Thumb (n=69) | Index finger (n=14) | Middle finger (n=58) | Ring finger (n=26) | Little finger (n=4) | Other fingers (n=102) | ||
| No. of injections | 0.409 | |||||||
| 1 | 120 (70.2) | 46 (66.7) | 10 (71.4) | 44 (75.9) | 17 (65.4) | 3 (75.0) | 74 (72.5) | |
| 2 | 51 (29.8) | 23 (33.3) | 4 (28.6) | 14 (24.1) | 9 (34.6) | 1 (25.0) | 28 (27.5) | |
| Response | 0.048 | |||||||
| Good/fair | 143 | 53 | 12 | 51 | 22 | 3 | 90 | |
| Poor | 28 | 16 | 2 | 7 | 4 | 1 | 12 | |
| Efficacy | 143 (83.6) | 53 (76.8) | 12 (85.7) | 51 (87.9) | 22 (84.6) | 3 (75.0) | 90 (88.2) | |
| Quinnell grade | 0.647 | |||||||
| II | 99 (57.9) | 37 (53.6) | 9 (64.3) | 36 (62.1) | 14 (53.8) | 3 (75.0) | 62 (60.8) | |
| III | 56 (32.7) | 25 (36.2) | 2 (14.3) | 21 (36.2) | 8 (30.8) | 0 (0) | 31 (30.4) | |
| IV | 16 (9.4) | 7 (10.1) | 1 (7.1) | 5 (8.6) | 2 (7.7) | 1 (25.0) | 9 (8.8) | |




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download