Abstract
Purpose
Dorsal metacarpal artery (DMA) flaps have been successfully used for distal dorsal finger defects. Some studies have reported inconsistent DMA anatomy, and there have been no studies on the anatomic variation of DMAs in Asian cadavers. Therefore, we evaluated the anatomy of the DMA using Korean fresh cadavers and reported the clinical outcomes of the DMA flaps.
Methods
In this cadaveric study, four human forearms from adult fresh cadavers were dissected. The dorsal metacarpal arteries and their communicating branches were identified. From July 2016 to June 2019, five patients with dorsal finger defects underwent a first DMA (FDMA) flap or a reverse DMA (RDMA) flap.
Results
In our cadaver study, the ulnar branch of the FDMA and the second and third DMAs were absent in two of four (50%) of the cadavers. In our case series, five flaps survived, and one had partial necrosis, which healed by the second intention. The mean operation time was approximately 100 minutes, and the mean outpatient follow-up period was 6 months.
Conclusion
DMA flaps are for the reconstruction of relatively large soft tissue defects of the dorsal finger. However, our anatomical study identified inconsistencies in the anatomy of DMAs. Therefore, a preoperative Doppler examination is required to evaluate the anatomy of the DMA before considering the use of a DMA flap.
The reconstruction of a distal finger injury is challenging for hand surgeons due to the complicated structure of the distal finger as well as the scarcity of the available surrounding tissue. Several surgical techniques, such as cross-finger flaps, thenar flaps, homodigital flaps, and free flaps, have been proposed to reconstruct distal dorsal finger defects [1]. However, these surgical techniques have several limitations. For example, a cross-finger flap has the advantages of easy harvesting and reliable anatomy but requires two operative stages and an immobilization period, which causes stiffness of the fingers. Free flaps can cover any size of a finger defect and can be used in any site of finger defects, but they require a long operation time and are difficult surgically.
Dorsal metacarpal artery (DMA) flaps, such as the first DMA (FDMA) flap and the reverse DMA (RDMA) flap, have been used successfully for distal dorsal finger defects [2,3]. They can cover relatively large wounds and can enable the early mobilization of the affected finger, and they also have consistent anatomy [4]. However, Sebastin et al. [5] noted that in their clinical study, the caliber of the DMAs decreased and the incidence of congenital absence of the DMA increased from the first dorsal metacarpal space to the fourth dorsal metacarpal space. Although there have been several anatomic studies of DMAs, they were evaluated in cadavers from the Western [6,7]. There have been no studies on the anatomic variation of DMAs in Asian cadavers. Therefore, the purposes of this study were to evaluate the anatomy of DMA using Korean fresh cadavers and to report the clinical outcomes of DMA flaps.
Ethics statements: This study was performed in accordance with the Declaration of Helsinki. The study was conducted after obtaining approval from the Institutional Review Board of Keimyung University School of Medicine (No. 2021-07-057).
Four human forearms from adult fresh cadavers were harvested from the Keimyung University School of Medicine. The mean age of the subjects was 74.5 years (70–79 years). We dissected two left and two right hands. An H-shaped dorsal cutaneous incision was made, and the cutaneous and subcutaneous tissues and the extensor apparatus were excised. The four intermetacarpal spaces were then dissected, and pictures of each intermetacarpal space were taken with a digital camera (Nikon D7500; Nikon, Tokyo, Japan).
We retrospectively reviewed patients who underwent FDMA flaps or RDMA flaps between July 2016 and June 2019. The FDMA flap was used to cover the dorsal thumb defect, and the RDMA flap was used to cover the dorsal defect of the index and ring fingers. All operations were performed by a single surgeon (JC). Medical charts were reviewed to obtain the demographic characteristics and complications.
After identifying the ulnar branch of the FDMA with Doppler ultrasound, the course of the ulnar branch of FDMA was marked with a marking pen. The flap was designed over the dorsum of the proximal phalanx of the index finger between the midaxial lines of the phalanx. The proximal extent of the island flap was at the point of the metacarpophalangeal (MCP) joint, and the distal extent was the proximal interphalangeal joint. A lazy “S” type incision was made from the head of the second metacarpal bone to the base of the first web space. The subcutaneous pedicle, including the ulnar branch of FDMA and the surrounding tissues, was dissected superficially to the paratenon. During dissection, the ulnar branch of FDMA was identified by Doppler ultrasonography. A tunnel was made toward the thumb, and then the flap was passed through the tunnel to cover the defect. The flaps were sutured with nylon. A full-thickness skin graft was used to close the donor site.
The flap was designed over the second, third, and fourth intermetacarpal spaces from the MCP joint to the distal wrist fold. After identifying the DMA with Doppler ultrasound, the course of the DMA was marked with a marking pen. The flap was then elevated in a proximal to distal direction and superficial to the paratenon. The flap’s pivot point was usually 0.5 to 1 cm proximal to the adjacent MCP joint, where there are distal connections between the palmar metacarpal arteries and the dorsal metacarpal arteries. If the donor site defect could not be closed with a primary closure, a skin graft was used to cover the defect.
The FDMA was found to arise from the radial artery in the first intermetacarpal space. It ran superficially over the first dorsal interosseous muscle and then divided into the radial branch to the thumb, the intermediate branch to the first web space, and the ulnar branch to the index finger. The ulnar branch ran distally between the shaft of the second metacarpal bone and the ulnar head of the first dorsal interosseous muscle until reaching the MCP joint (Fig. 1). The ulnar branch of the FDMA was absent in two of the four cadavers (Fig. 2). The second, third, and fourth DMAs were identified over the epimysium of the dorsal interosseous muscles and arose from the dorsal metacarpal arch. The Juncturae tendinum was removed to identify the course of the arteries. The second and third DMAs were not identified in the two cases (Fig. 3). In one case, we observed a connection between the third DMA and the palmar carpal arch at the metacarpal shaft (Fig. 4).
Five patients were included; four men and one woman, with a mean age of 58.6 years (range, 34–69 years). All reconstructions were performed under general anesthesia. The defects were in the dorsal thumb (n=4), dorsal index finger (n=1), and dorsal middle finger (n=1). The causes of the defects were squamous cell carcinoma (n=2), malignant melanoma (n=1), a posttraumatic defect (n=1), and secondary to a burn (n=1). Four FDMA flaps and two RDMA flaps were performed. The average flap size was 3.4×2 cm2 (range, 3×1.8–4×2.5 cm2). Five flaps had complete healing, and one FDMA flap had partial necrosis, which healed by the second intention. The mean operation time was approximately 100 minutes, and the mean outpatient follow-up period was 6 months. All donor sites were treated with a skin graft (Table 1; Fig. 5, 6).
In our study, five flaps had complete healing, and one flap had partial necrosis, which healed by the second intention. Several studies that evaluated the use of thumb reconstruction with FDMA flaps reported a 90% to 100% flap survival rate [8-10], and several studies that evaluated RDMA flaps also reported that the flap survival rates were nearly 88% to 93% [2,11]. These favorable results support the hypothesis that FDMA and RDMA flaps are reliable reconstruction methods for relatively large distal dorsal finger defects. We could identify that the DMAs have constant anatomy, running over the epimysium of the dorsal interosseous muscles, reliable as a source of pedicle flaps.
However, in our cadaver study, the ulnar branch of the FDMA was not identified in two of the four cadavers (50.0%). This rate was higher than that in other FDMA cadaveric studies. Murakami et al. [12] reported that the ulnar branch of the FDMA was not observed in 14 of 62 cadaveric hands (22.5%). Sherif [13] reported that in their clinical study, the ulnar branch of the FDMA was not observed in four of 18 patients (22.2%). Therefore, the higher rate in our cadaver study may be attributed to the small number of specimens. Since the FDMA could be congenitally absent, it is very important to check for the presence of the ulnar branch of the FDMA preoperatively by using Doppler ultrasound. In our study, the second and third DMAs were not identified in two of the four cadavers (50.0%). Beldame et al. [14] reported that in their cadaver study, the third and fourth DMAs were not observed in 15.3% and 57.2% of the cadavers, respectively [14], and Dauphin and Casoli [6] reported that in their cadaver study, the third and fourth DMAs were not observed in 3% and 14% of the cadavers, respectively. Sebastin et al. [5] and Couceiro et al. [15] also noted that a preoperative Doppler examination is mandatory when considering the use of DMA flaps. As we mentioned earlier, in our study, the Doppler ultrasound was performed before every operation and the success rate of the operation was also high. If there is no ulnar branch of FDMA at preoperative check, it is recommended to consider a free flap.
Ghoraba and Mahmoud [8] noted that the FDMA has two venae comitantes that usually have a connection with the large cutaneous superficial veins. In many of the studies that have used FDMA flaps, one or two dorsal cutaneous veins were included in the subcutaneous pedicle to prevent venous pathologies. However, while some articles have proposed that the superficial dorsal vein should be included in the pedicle [16,17], in other studies, it was not essential [10,18]. In our two patients that had FDMA flaps, the superficial dorsal vein was included in the pedicle, and in the other two patients, it was not included. In cases that did not include the superficial dorsal vein in the pedicle, the flaps healed well without venous pathologies. Thus, venous complications may not be associated with the inclusion of the superficial dorsal veins with the subcutaneous pedicle. Meanwhile, since venae comitantes are very small and thin, they can be easily damaged when dividing the superficial dorsal veins from the subcutaneous pedicle if the superficial dorsal vein has the same course as the pedicle. Therefore, in such a case, it would be better to include the dorsal vein within the pedicle.
Generally, the RDMA flap’s pivot point is usually 0.5 to 1 cm proximal to the adjacent MCP joint, where there are distal connections between the palmar metacarpal arteries and the dorsal metacarpal arteries [19]. Omokawa et al. [20] reported that the DMAs were consistently connected to the palmar arterial system at the level of the metacarpal head; however, some articles have reported that the connection of the DMAs and the palmar arterial system was observed to be more proximal to the level of the MCP joint. Yoon et al. noted that the fourth DMA anastomoses with the vessels branching from the deep palmar arch at the level of the metacarpal shaft [21]. Dauphin and Casoli [6] reported that palmar perforators anastomose with the DMA at the proximal part of the intermetacarpal space. In our cadaver study, we also observed the connection between the third DMA and the deep palmar arch vessels at the level of the metacarpal shaft. The connecting vessels are very small and can be easily torn during dissection. When the branching vessels are injured, the vessel pedicle can also be damaged during hemostasis. Therefore, when elevating an RDMA flap, careful dissection is necessary during elevating the flap proximal to the distal direction, until it reaches the MCP joint.
There are several limitations to our study. First, it was a retrospectively designed study, and second, our study was a small-scale study. Third, the mean follow-up period was 6 months, which was very short to evaluate any long-term complications such as scar contracture. Despite these disadvantages, to the best of our knowledge, this is the first study to evaluate the DMA anatomy using Asian fresh cadavers.
The good surgical results that were found in this study showed that DMA flaps are a reliable flap for the reconstruction of relatively large soft tissue defects of the dorsal finger. The absence of the DMAs in some cadavers was noted in our cadaver study. Therefore, a preoperative Doppler examination is mandatory when considering the use of DMA flaps.
References
3. Foucher G, Braun JB. A new island flap transfer from the dorsum of the index to the thumb. Plast Reconstr Surg. 1979; 63:344–9.

4. de Rezende MR, Mattar Júnior R, Cho AB, Hasegawa OH, Ribak S. Anatomic study of the dorsal arterial system of the hand. Rev Hosp Clin Fac Med Sao Paulo. 2004; 59:71–6.

5. Sebastin SJ, Mendoza RT, Chong AK, et al. Application of the dorsal metacarpal artery perforator flap for resurfacing soft-tissue defects proximal to the fingertip. Plast Reconstr Surg. 2011; 128:166e–178e.

6. Dauphin N, Casoli V. The dorsal metacarpal arteries: anatomical study: feasibility of pedicled metacarpal bone flaps. J Hand Surg Eur Vol. 2011; 36:787–94.

7. Earley MJ. The arterial supply of the thumb, first web and index finger and its surgical application. J Hand Surg Br. 1986; 11:163–74.

8. Ghoraba SM, Mahmoud WH. Outcome of thumb reconstruction using the first dorsal metacarpal artery island flap. World J Plast Surg. 2018; 7:151–8.
9. Satish C, Nema S. First dorsal metacarpal artery islanded flap: a useful flap for reconstruction of thumb pulp defects. Indian J Plast Surg. 2009; 42:32–5.

10. Zhang X, Shao X, Ren C, Zhang Z, Wen S, Sun J. Reconstruction of thumb pulp defects using a modified kite flap. J Hand Surg Am. 2011; 36:1597–603.

11. Balan JR, Mathew S, Kumar P, et al. The reverse dorsal metacarpal artery flap in finger reconstruction: a reliable choice. Indian J Plast Surg. 2018; 51:54–9.

12. Murakami T, Takaya K, Outi H. The origin, course and distribution of arteries to the thumb, with special reference to the so-called A. princeps pollicis. Okajimas Folia Anat Jpn. 1969; 46:123–37.

13. Sherif MM. First dorsal metacarpal artery flap in hand reconstruction. I. Anatomical study. J Hand Surg Am. 1994; 19:26–31.

14. Beldame J, Havet E, Auquit-Auckbur I, Lefebvre B, Mure JP, Duparc F. Arterial anatomical basis of the dorsal digito-metacarpal flap for long fingers. Surg Radiol Anat. 2008; 30:429–35.

15. Couceiro J, de Prado M, Menendez G, Manteiga Z. The first dorsal metacarpal artery flap family: a review. Surg J (N Y). 2018; 4:e215–9.

16. Eski M, Nisanci M, Sengezer M. Correction of thumb deformities after burn: versatility of first dorsal metacarpal artery flap. Burns. 2007; 33:65–71.

17. Karacalar A, Ozcan M. A new approach to the reverse dorsal metacarpal artery flap. J Hand Surg Am. 1997; 22:307–10.

18. Ratcliffe RJ, Regan PJ, Scerri GV. First dorsal metacarpal artery flap cover for extensive pulp defects in the normal length thumb. Br J Plast Surg. 1992; 45:544–6.

Fig. 1.
Diagrammatic presentation of the first dorsal metacarpal artery (FDMA) and its three branches.
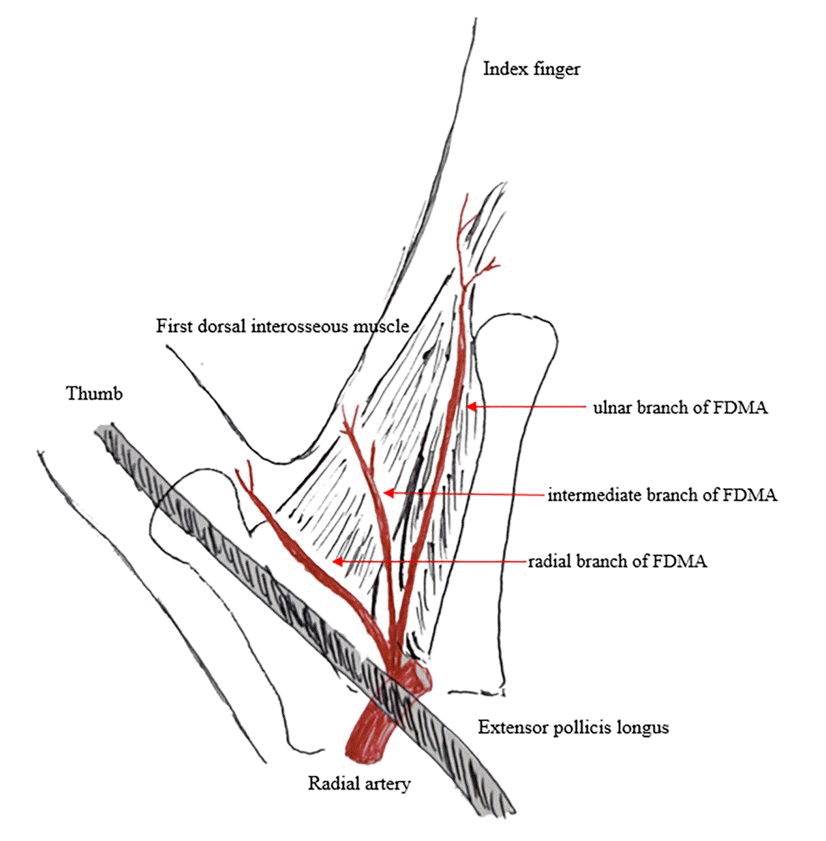
Fig. 2.
A right hand: on the upper side of the figure, a global view; on the lower side, a detailed view of the first intermetacarpal space. The yellow arrow indicates the absence of the ulnar branch of the first dorsal metacarpal artery.
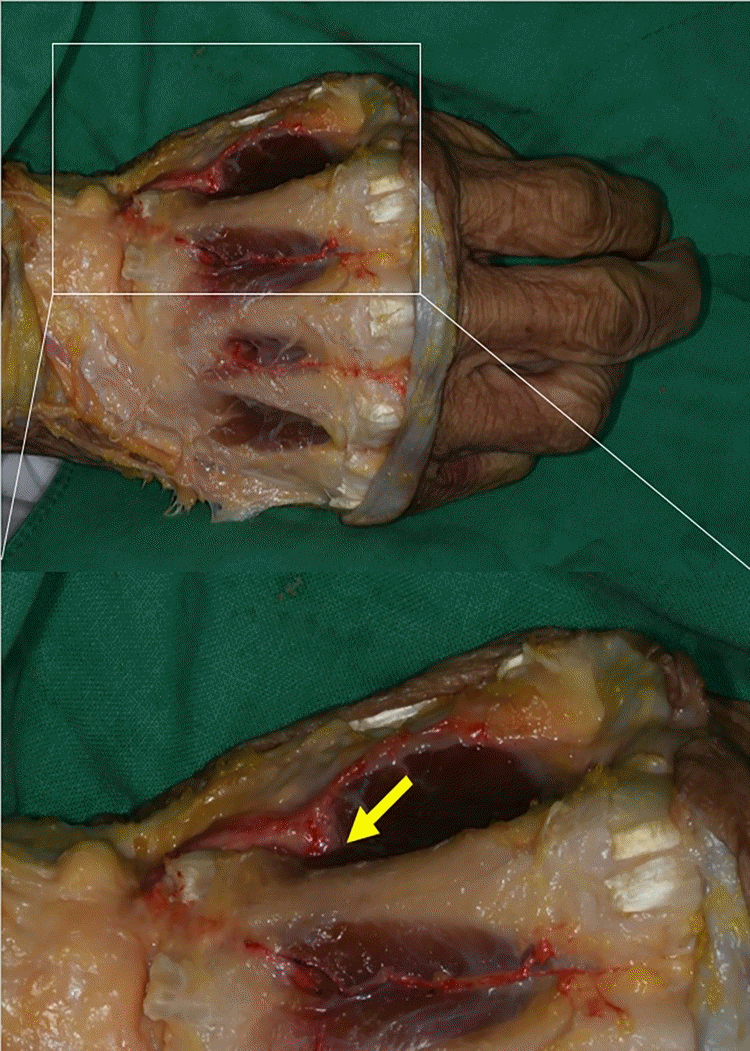
Fig. 3.
Absent dorsal metacarpal arteries. The red arrows point to the absent second dorsal metacarpal artery (A) and third dorsal metacarpal artery (B) in each intermetacarpal area.
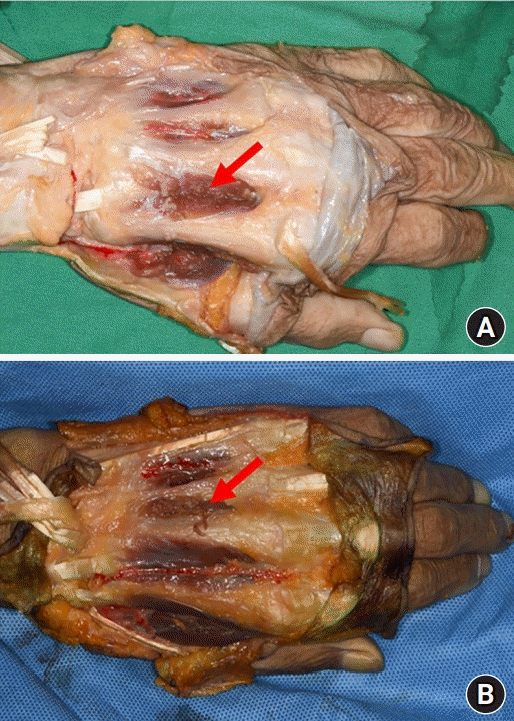
Fig. 4.
The red arrow points to the communication of the dorsal metacarpal artery and the palmar arch.
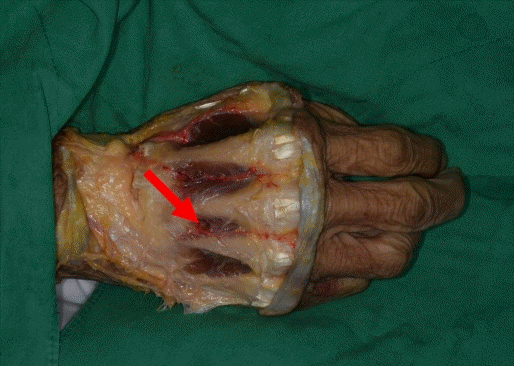
Fig. 5.
Case 1. A 69-year-old woman with squamous cell carcinoma on the nail bed of the right thumb. (A) A thumb nail bed defect. A first dorsal metacarpal artery flap, 3.0×1.8 cm2, was elevated on the dorsum of the index finger. (B) The defect was covered with a flap. (C) One-month postoperative view.
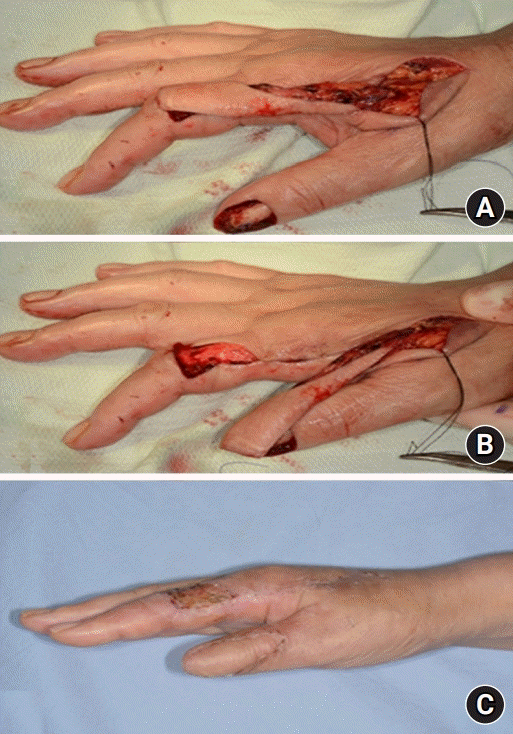
Fig. 6.
Case 2. A 34-year-old man with a contact burn, extending from the left thumb to the middle finger. (A, B) The skin defect at the tip of the left thumb and ring finger. (C) Reverse dorsal metacarpal artery flap, 4.0×2.5 cm2, from the third-fourth intermetacarpal space covering the tip of the ring finger. A first dorsal metacarpal artery flap, 3.2×2.0 cm2, covered the defect on the tip of the thumb. (D) Two-month postoperative view.
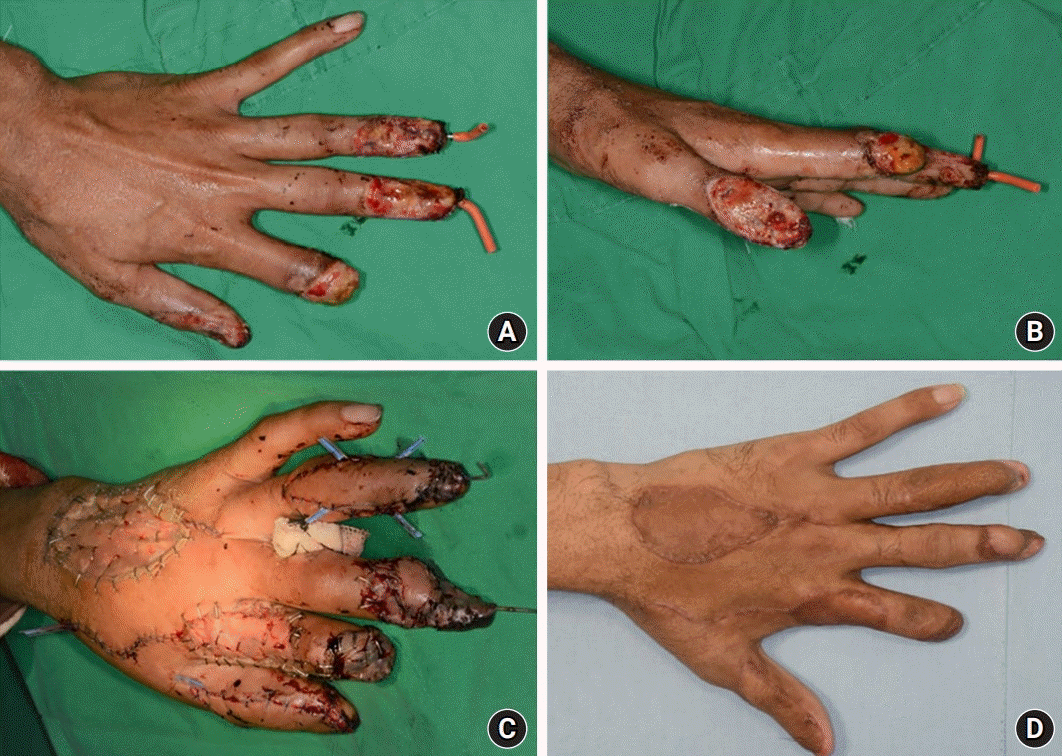
Table 1.
Patient demographics




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download