1. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017; 390:2627–42.
2. Hruby A, Hu FB. The epidemiology of obesity: a big picture. Pharmacoeconomics. 2015; 33:673–89.

3. Jung CH, Son JW, Kang S, Kim WJ, Kim HS, Kim HS, et al. Diabetes fact sheets in Korea, 2020: an appraisal of current status. Diabetes Metab J. 2021; 45:1–10.

4. Celik O, Yildiz BO. Obesity and physical exercise. Minerva Endocrinol (Torino). 2021; 46:131–44.

5. Goodpaster BH, Sparks LM. Metabolic flexibility in health and disease. Cell Metab. 2017; 25:1027–36.

6. Russell AP, Foletta VC, Snow RJ, Wadley GD. Skeletal muscle mitochondria: a major player in exercise, health and disease. Biochim Biophys Acta. 2014; 1840:1276–84.

7. Huertas JR, Casuso RA, Agustin PH, Cogliati S. Stay fit, stay young: mitochondria in movement: the role of exercise in the new mitochondrial paradigm. Oxid Med Cell Longev. 2019; 2019:7058350.

8. Hood DA, Memme JM, Oliveira AN, Triolo M. Maintenance of skeletal muscle mitochondria in health, exercise, and aging. Annu Rev Physiol. 2019; 81:19–41.

9. Lundby C, Jacobs RA. Adaptations of skeletal muscle mitochondria to exercise training. Exp Physiol. 2016; 101:17–22.

10. Szendroedi J, Phielix E, Roden M. The role of mitochondria in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol. 2011; 8:92–103.

11. Bishop DJ, Botella J, Genders AJ, Lee MJ, Saner NJ, Kuang J, et al. High-intensity exercise and mitochondrial biogenesis: current controversies and future research directions. Physiology (Bethesda). 2019; 34:56–70.

12. Austin S, St-Pierre J. PGC1α and mitochondrial metabolism: emerging concepts and relevance in ageing and neurodegenerative disorders. J Cell Sci. 2012; 125(Pt 21):4963–71.
13. Tadaishi M, Miura S, Kai Y, Kano Y, Oishi Y, Ezaki O. Skeletal muscle-specific expression of PGC-1α-b, an exercise-responsive isoform, increases exercise capacity and peak oxygen uptake. PLoS One. 2011; 6:e28290.

14. Scarpulla RC. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim Biophys Acta. 2011; 1813:1269–78.

15. Miura S, Kawanaka K, Kai Y, Tamura M, Goto M, Shiuchi T, et al. An increase in murine skeletal muscle peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1alpha) mRNA in response to exercise is mediated by beta-adrenergic receptor activation. Endocrinology. 2007; 148:3441–8.
16. Maier LS, Bers DM. Role of Ca
2+/calmodulin-dependent protein kinase (CaMK) in excitation-contraction coupling in the heart. Cardiovasc Res. 2007; 73:631–40.

17. Rose AJ, Hargreaves M. Exercise increases Ca
2+-calmodulin-dependent protein kinase II activity in human skeletal muscle. J Physiol. 2003; 553(Pt 1):303–9.

18. Rose AJ, Kiens B, Richter EA. Ca
2+-calmodulin-dependent protein kinase expression and signalling in skeletal muscle during exercise. J Physiol. 2006; 574(Pt 3):889–903.

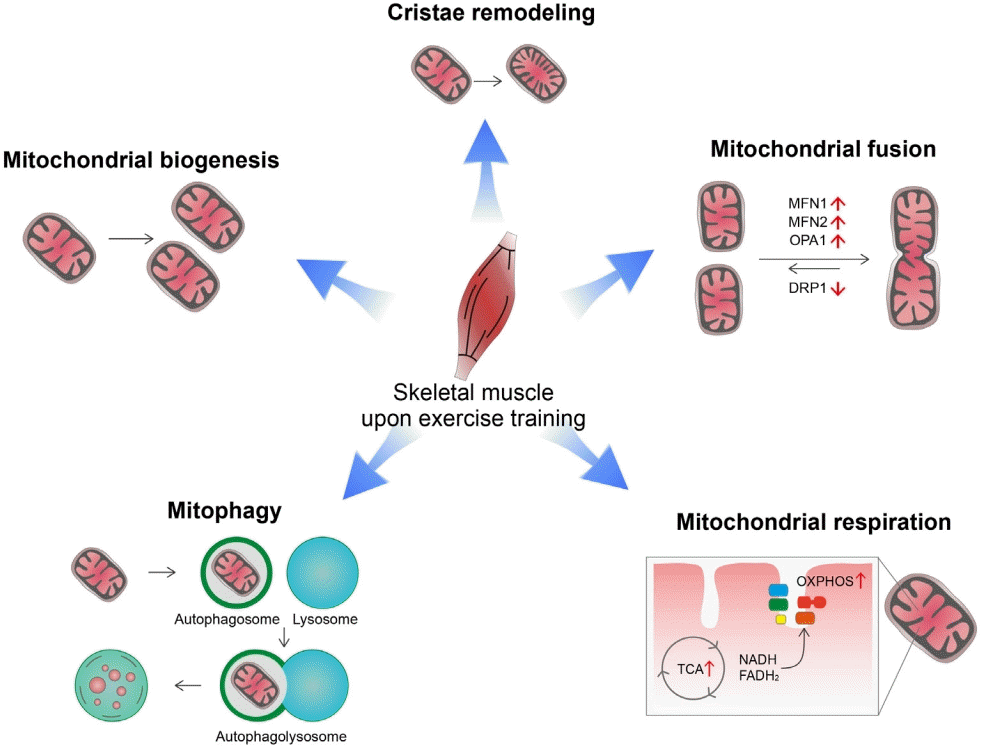
19. Wu H, Kanatous SB, Thurmond FA, Gallardo T, Isotani E, Bassel-Duby R, et al. Regulation of mitochondrial biogenesis in skeletal muscle by CaMK. Science. 2002; 296:349–52.

20. Joseph JS, Anand K, Malindisa ST, Oladipo AO, Fagbohun OF. Exercise, CaMKII, and type 2 diabetes. EXCLI J. 2021; 20:386–99.
21. Nielsen J, Gejl KD, Hey-Mogensen M, Holmberg HC, Suetta C, Krustrup P, et al. Plasticity in mitochondrial cristae density allows metabolic capacity modulation in human skeletal muscle. J Physiol. 2017; 595:2839–47.

22. Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012; 337:1062–5.

23. Lee JE, Westrate LM, Wu H, Page C, Voeltz GK. Multiple dynamin family members collaborate to drive mitochondrial division. Nature. 2016; 540:139–43.

24. Ishihara N, Eura Y, Mihara K. Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity. J Cell Sci. 2004; 117(Pt 26):6535–46.

25. Mishra P, Chan DC. Metabolic regulation of mitochondrial dynamics. J Cell Biol. 2016; 212:379–87.

26. Cartoni R, Leger B, Hock MB, Praz M, Crettenand A, Pich S, et al. Mitofusins 1/2 and ERRalpha expression are increased in human skeletal muscle after physical exercise. J Physiol. 2005; 567(Pt 1):349–58.
27. Bell MB, Bush Z, McGinnis GR, Rowe GC. Adult skeletal muscle deletion of Mitofusin 1 and 2 impedes exercise performance and training capacity. J Appl Physiol (1985). 2019; 126:341–53.

28. Boya P, Reggiori F, Codogno P. Emerging regulation and functions of autophagy. Nat Cell Biol. 2013; 15:713–20.

29. Guan Y, Drake JC, Yan Z. Exercise-induced mitophagy in skeletal muscle and heart. Exerc Sport Sci Rev. 2019; 47:151–6.

30. Yun J, Finkel T. Mitohormesis. Cell Metab. 2014; 19:757–66.

31. Ristow M, Zarse K. How increased oxidative stress promotes longevity and metabolic health: the concept of mitochondrial hormesis (mitohormesis). Exp Gerontol. 2010; 45:410–8.

32. Cox CS, McKay SE, Holmbeck MA, Christian BE, Scortea AC, Tsay AJ, et al. Mitohormesis in mice via sustained basal activation of mitochondrial and antioxidant signaling. Cell Metab. 2018; 28:776–86.

33. Musci RV, Hamilton KL, Linden MA. Exercise-induced mitohormesis for the maintenance of skeletal muscle and healthspan extension. Sports (Basel). 2019; 7:170.

34. Kojda G, Harrison D. Interactions between NO and reactive oxygen species: pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc Res. 1999; 43:562–71.

35. Wiseman H, Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem J. 1996; 313(Pt 1):17–29.

36. Bennett S, Grant MM, Aldred S. Oxidative stress in vascular dementia and Alzheimer’s disease: a common pathology. J Alzheimers Dis. 2009; 17:245–57.

37. Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, Kiehntopf M, et al. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009; 106:8665–70.

38. Kang GM, Min SH, Lee CH, Kim JY, Lim HS, Choi MJ, et al. Mitohormesis in hypothalamic POMC neurons mediates regular exercise-induced high-turnover metabolism. Cell Metab. 2021; 33:334–49.

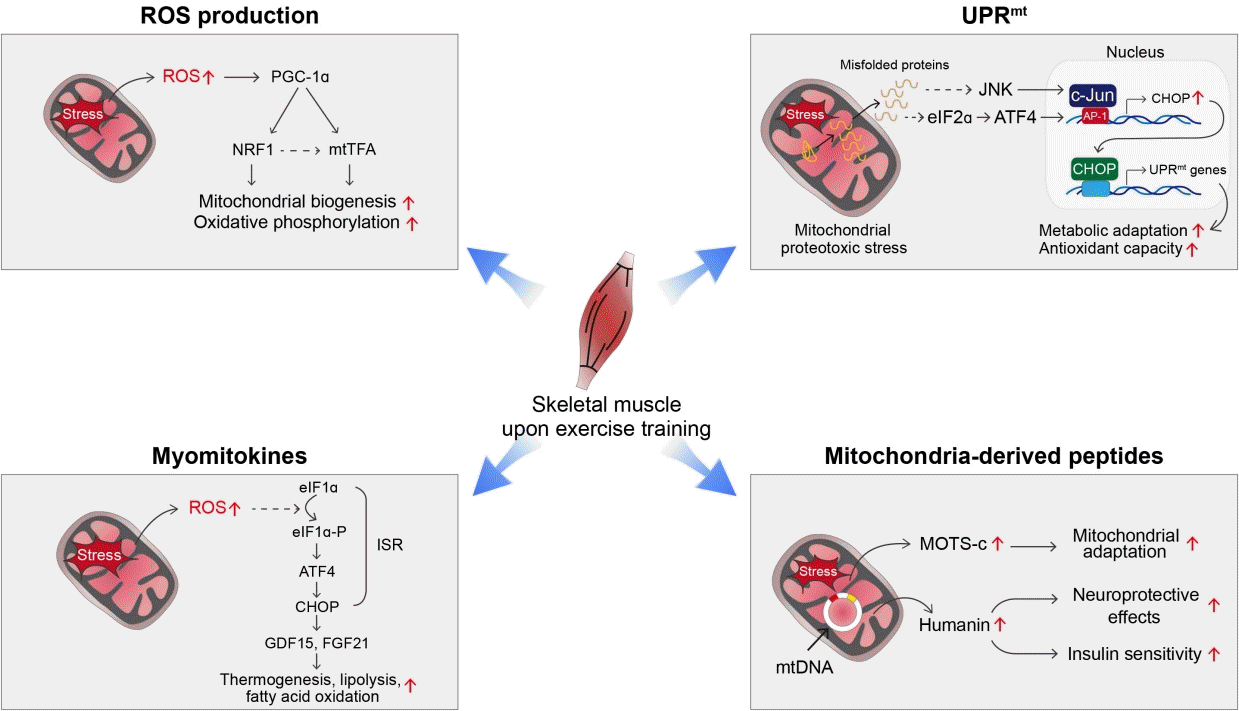
39. Shpilka T, Haynes CM. The mitochondrial UPR: mechanisms, physiological functions and implications in ageing. Nat Rev Mol Cell Biol. 2018; 19:109–20.

40. Mottis A, Herzig S, Auwerx J. Mitocellular communication: shaping health and disease. Science. 2019; 366:827–32.

41. Qureshi MA, Haynes CM, Pellegrino MW. The mitochondrial unfolded protein response: signaling from the powerhouse. J Biol Chem. 2017; 292:13500–6.

42. Durieux J, Wolff S, Dillin A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell. 2011; 144:79–91.

43. Cordeiro AV, Peruca GF, Braga RR, Bricola RS, Lenhare L, Silva V, et al. High-intensity exercise training induces mitonuclear imbalance and activates the mitochondrial unfolded protein response in the skeletal muscle of aged mice. Geroscience. 2021; 43:1513–8.

44. Laurens C, Parmar A, Murphy E, Carper D, Lair B, Maes P, et al. Growth and differentiation factor 15 is secreted by skeletal muscle during exercise and promotes lipolysis in humans. JCI Insight. 2020; 5:e131870.

45. Kim KH, Kim SH, Min YK, Yang HM, Lee JB, Lee MS. Acute exercise induces FGF21 expression in mice and in healthy humans. PLoS One. 2013; 8:e63517.

46. Johann K, Kleinert M, Klaus S. The role of GDF15 as a myomitokine. Cells. 2021; 10:2990.

47. Kim KH, Lee MS. FGF21 as a stress hormone: the roles of FGF21 in stress adaptation and the treatment of metabolic diseases. Diabetes Metab J. 2014; 38:245–51.

48. Kim KH, Lee MS. GDF15 as a central mediator for integrated stress response and a promising therapeutic molecule for metabolic disorders and NASH. Biochim Biophys Acta Gen Subj. 2021; 1865:129834.

49. Woodhead J, Merry TL. Mitochondrial-derived peptides and exercise. Biochim Biophys Acta Gen Subj. 2021; 1865:130011.

50. Merry TL, Chan A, Woodhead J, Reynolds JC, Kumagai H, Kim SJ, et al. Mitochondrial-derived peptides in energy metabolism. Am J Physiol Endocrinol Metab. 2020; 319:E659–66.

51. Bodzioch M, Lapicka-Bodzioch K, Zapala B, Kamysz W, Kiec-Wilk B, Dembinska-Kiec A. Evidence for potential functionality of nuclearly-encoded humanin isoforms. Genomics. 2009; 94:247–56.

52. Hashimoto Y, Niikura T, Tajima H, Yasukawa T, Sudo H, Ito Y, et al. A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer’s disease genes and Abeta. Proc Natl Acad Sci U S A. 2001; 98:6336–41.
53. Hashimoto Y, Niikura T, Ito Y, Sudo H, Hata M, Arakawa E, et al. Detailed characterization of neuroprotection by a rescue factor humanin against various Alzheimer’s disease-relevant insults. J Neurosci. 2001; 21:9235–45.

54. Ikonen M, Liu B, Hashimoto Y, Ma L, Lee KW, Niikura T, et al. Interaction between the Alzheimer’s survival peptide humanin and insulin-like growth factor-binding protein 3 regulates cell survival and apoptosis. Proc Natl Acad Sci U S A. 2003; 100:13042–7.

55. Hashimoto Y, Tsuji O, Niikura T, Yamagishi Y, Ishizaka M, Kawasumi M, et al. Involvement of c-Jun N-terminal kinase in amyloid precursor protein-mediated neuronal cell death. J Neurochem. 2003; 84:864–77.

56. Kuliawat R, Klein L, Gong Z, Nicoletta-Gentile M, Nemkal A, Cui L, et al. Potent humanin analog increases glucose-stimulated insulin secretion through enhanced metabolism in the β cell. FASEB J. 2013; 27:4890–8.

57. Muzumdar RH, Huffman DM, Atzmon G, Buettner C, Cobb LJ, Fishman S, et al. Humanin: a novel central regulator of peripheral insulin action. PLoS One. 2009; 4:e6334.

58. Cobb LJ, Lee C, Xiao J, Yen K, Wong RG, Nakamura HK, et al. Naturally occurring mitochondrial-derived peptides are age-dependent regulators of apoptosis, insulin sensitivity, and inflammatory markers. Aging (Albany NY). 2016; 8:796–809.

59. Lee C, Zeng J, Drew BG, Sallam T, Martin-Montalvo A, Wan J, et al. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab. 2015; 21:443–54.

60. Bhullar KS, Shang N, Kerek E, Wu K, Wu J. Mitofusion is required for MOTS-c induced GLUT4 translocation. Sci Rep. 2021; 11:14291.

61. Yin Y, Pan Y, He J, Zhong H, Wu Y, Ji C, et al. The mitochondrial-derived peptide MOTS-c relieves hyperglycemia and insulin resistance in gestational diabetes mellitus. Pharmacol Res. 2022; 175:105987.

62. Kong BS, Min SH, Lee C, Cho YM. Mitochondrial-encoded MOTS-c prevents pancreatic islet destruction in autoimmune diabetes. Cell Rep. 2021; 36:109447.

63. Wei M, Gan L, Liu Z, Liu L, Chang JR, Yin DC, et al. Mitochondrial-derived peptide MOTS-c attenuates vascular calcification and secondary myocardial remodeling via adenosine monophosphate-activated protein kinase signaling pathway. Cardiorenal Med. 2020; 10:42–50.

64. Yan Z, Zhu S, Wang H, Wang L, Du T, Ye Z, et al. MOTS-c in hibits osteolysis in the mouse calvaria by affecting osteocyte-osteoclast crosstalk and inhibiting inflammation. Pharmacol Res. 2019; 147:104381.
65. Ming W, Lu G, Xin S, Huanyu L, Yinghao J, Xiaoying L, et al. Mitochondria related peptide MOTS-c suppresses ovariectomy-induced bone loss via AMPK activation. Biochem Biophys Res Commun. 2016; 476:412–9.

66. Yin X, Jing Y, Chen Q, Abbas AB, Hu J, Xu H. The intraperitoneal administration of MOTS-c produces antinociceptive and anti-inflammatory effects through the activation of AMPK pathway in the mouse formalin test. Eur J Pharmacol. 2020; 870:172909.

67. Woodhead J, D’Souza RF, Hedges CP, Wan J, Berridge MV, Cameron-Smith D, et al. High-intensity interval exercise increases humanin, a mitochondrial encoded peptide, in the plasma and muscle of men. J Appl Physiol (1985). 2020; 128:1346–54.

68. Reynolds JC, Lai RW, Woodhead J, Joly JH, Mitchell CJ, Cameron-Smith D, et al. MOTS-c is an exercise-induced mitochondrial-encoded regulator of age-dependent physical decline and muscle homeostasis. Nat Commun. 2021; 12:470.

69. von Walden F, Fernandez-Gonzalo R, Norrbom J, Emanuelsson EB, Figueiredo VC, Gidlund EK, et al. Acute endurance exercise stimulates circulating levels of mitochondrial-derived peptides in humans. J Appl Physiol (1985). 2021; 131:1035–42.

70. Gidlund EK, von Walden F, Venojarvi M, Riserus U, Heinonen OJ, Norrbom J, et al. Humanin skeletal muscle protein levels increase after resistance training in men with impaired glucose metabolism. Physiol Rep. 2016; 4:e13063.

71. Dieli-Conwright CM, Sami N, Norris MK, Wan J, Kumagai H, Kim SJ, et al. Effect of aerobic and resistance exercise on the mitochondrial peptide MOTS-c in Hispanic and non-Hispanic White breast cancer survivors. Sci Rep. 2021; 11:16916.

72. Ramanjaneya M, Jerobin J, Bettahi I, Bensila M, Aye M, Siveen KS, et al. Lipids and insulin regulate mitochondrial-derived peptide (MOTS-c) in PCOS and healthy subjects. Clin Endocrinol (Oxf). 2019; 91:278–87.

73. Yang B, Yu Q, Chang B, Guo Q, Xu S, Yi X, et al. MOTS-c interacts synergistically with exercise intervention to regulate PGC-1α expression, attenuate insulin resistance and enhance glucose metabolism in mice via AMPK signaling pathway. Biochim Biophys Acta Mol Basis Dis. 2021; 1867:166126.

74. Guo Q, Chang B, Yu QL, Xu ST, Yi XJ, Cao SC. Adiponectin treatment improves insulin resistance in mice by regulating the expression of the mitochondrial-derived peptide MOTS-c and its response to exercise via APPL1-SIRT1-PGC-1α. Diabetologia. 2020; 63:2675–88.

75. Niikura T, Hashimoto Y, Tajima H, Ishizaka M, Yamagishi Y, Kawasumi M, et al. A tripartite motif protein TRIM11 binds and destabilizes humanin, a neuroprotective peptide against Alzheimer’s disease-relevant insults. Eur J Neurosci. 2003; 17:1150–8.

76. Alves de Souza RW, Gallo D, Lee GR, Katsuyama E, Schaufler A, Weber J, et al. Skeletal muscle heme oxygenase-1 activity regulates aerobic capacity. Cell Rep. 2021; 35:109018.

77. Done AJ, Traustadottir T. Nrf2 mediates redox adaptations to exercise. Redox Biol. 2016; 10:191–9.

78. Merry TL, Ristow M. Nuclear factor erythroid-derived 2-like 2 (NFE2L2, Nrf2) mediates exercise-induced mitochondrial biogenesis and the anti-oxidant response in mice. J Physiol. 2016; 594:5195–207.
79. Flockhart M, Nilsson LC, Tais S, Ekblom B, Apro W, Larsen FJ. Excessive exercise training causes mitochondrial functional impairment and decreases glucose tolerance in healthy volunteers. Cell Metab. 2021; 33:957–70.
80. Kim KH, Son JM, Benayoun BA, Lee C. The mitochondrial-encoded peptide MOTS-c translocates to the nucleus to regulate nuclear gene expression in response to metabolic stress. Cell Metab. 2018; 28:516–24.
81. Kumagai H, Coelho AR, Wan J, Mehta HH, Yen K, Huang A, et al. MOTS-c reduces myostatin and muscle atrophy signaling. Am J Physiol Endocrinol Metab. 2021; 320:E680–90.

82. Lee C, Kim KH, Cohen P. MOTS-c: a novel mitochondrial-derived peptide regulating muscle and fat metabolism. Free Radic Biol Med. 2016; 100:182–7.

83. Lu H, Wei M, Zhai Y, Li Q, Ye Z, Wang L, et al. MOTS-c peptide regulates adipose homeostasis to prevent ovariectomy-induced metabolic dysfunction. J Mol Med (Berl). 2019; 97:473–85.

84. Wu MV, Bikopoulos G, Hung S, Ceddia RB. Thermogenic capacity is antagonistically regulated in classical brown and white subcutaneous fat depots by high fat diet and endurance training in rats: impact on whole-body energy expenditure. J Biol Chem. 2014; 289:34129–40.
85. Lehnig AC, Dewal RS, Baer LA, Kitching KM, Munoz VR, Arts PJ, et al. Exercise training induces depot-specific adaptations to white and brown adipose tissue. iScience. 2019; 11:425–39.

86. Periasamy M, Herrera JL, Reis F. Skeletal muscle thermogenesis and its role in whole body energy metabolism. Diabetes Metab J. 2017; 41:327–36.

87. Vidal P, Stanford KI. Exercise-induced adaptations to adipose tissue thermogenesis. Front Endocrinol (Lausanne). 2020; 11:270.

88. Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012; 481:463–8.

89. Allen DL, Hittel DS, McPherron AC. Expression and function of myostatin in obesity, diabetes, and exercise adaptation. Med Sci Sports Exerc. 2011; 43:1828–35.

90. Alizadeh H. Myokine-mediated exercise effects: the role of myokine meteorin-like hormone (Metrnl). Growth Factors. 2022; 39:71–8.

91. Mendez-Gutierrez A, Aguilera CM, Osuna-Prieto FJ, Martinez-Tellez B, Rico Prados MC, Acosta FM, et al. Exercise-induced changes on exerkines that might influence brown adipose tissue metabolism in young sedentary adults. Eur J Sport Sci. 2022; Apr. 25. [Epub].
https://doi.org/10.1080/17461391.2022.2040597.

92. Colitti M, Boschi F, Montanari T. Dynamic of lipid droplets and gene expression in response to β-aminoisobutyric acid treatment on 3T3-L1 cells. Eur J Histochem. 2018; 62:2984.

93. Lu H, Tang S, Xue C, Liu Y, Wang J, Zhang W, et al. Mitochondrial-derived peptide MOTS-c increases adipose thermogenic activation to promote cold adaptation. Int J Mol Sci. 2019; 20:2456.

94. Cordeiro AV, Bricola RS, Braga RR, Lenhare L, Silva V, Anaruma CP, et al. Aerobic exercise training induces the mitonuclear imbalance and UPRmt in the skeletal muscle of aged mice. J Gerontol A Biol Sci Med Sci. 2020; 75:2258–61.
95. Braga RR, Crisol BM, Bricola RS, Sant’ana MR, Nakandakari S, Costa SO, et al. Exercise alters the mitochondrial proteostasis and induces the mitonuclear imbalance and UPRmt in the hypothalamus of mice. Sci Rep. 2021; 11:3813.
96. Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001; 108:1167–74.






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download