INTRODUCTION
Progressive supranuclear palsy (PSP) closely overlaps with corticobasal degeneration (CBD) in terms of atypical parkinsonism and tauopathy.
1 The clinical features of CBD include asymmetric parkinsonism (rigidity, akinesia, myoclonus, and dystonia) and cortical symptoms (apraxia, alien hand, frontal lobe dementia, and aphasia).
2 PSP clinically presents as an early onset of unexplained frequent falls, subsequent parkinsonism, vertical gaze palsy, dementia, pseudobulbar palsy, and cervical dystonia.
3
Tau protein is a microtubule-associated phosphoprotein.
14 Six isoforms of tau proteins are generated by alternative mRNA splicing of exons 2, 3, and 10 in the adult human brain.
14 Tauopathies are classified into three repeat (exon 10 missing, 3R), four repeat (exon 10 present, 4R), or equal ratios of 3R and 4R tau due to the predominance of tau isoforms in cytoplasmic inclusions.
45 PSP and CBD present as an accumulation of abnormal 4R tau isoforms in glial cells and neurons.
1467
Regarding the misdiagnosis rate by the clinical–pathological overlapping between CBD and PSP, 42% of the pathologically diagnosed CBD cases clinically presented as a progressive supranuclear palsy and 29% of cases with corticobasal syndrome (CBS) had underlying PSP pathology.
8 Other studies have also shown that about a third of autopsy-proven CBD cases were clinically diagnosed as PSP.
28 Conversely, in autopsy-confirmed PSP cases, CBS presentation was rare.
89
Herein, we report the neuropathological features of a patient with autopsy-confirmed CBD with PSP–CBD overlapping symptoms.
DISCUSSION
The Movement Disorder Society Clinical Diagnostic Criteria for PSP (MDS-PSP) suggested the combinations of core clinical features, supportive clinical clues, and imaging findings plus the mandatory inclusion criteria of basic features.
3 The mandatory basic features were sporadic occurrence, age ≥ 40 years at onset, and gradual progression of PSP-related symptoms.
3 The four core features were ocular motor dysfunction, postural instability, akinesia, and cognitive dysfunction.
3 According to the level of certainty (highest, mild, and lowest) of the MDS-PSP criteria,
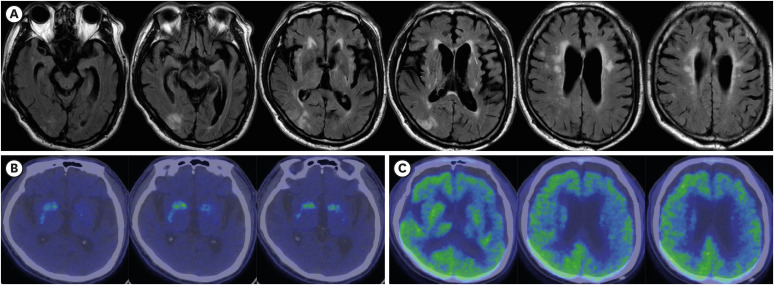
3 the initial symptoms of our patient corresponded to probable PSP because of the core features (vertical supranuclear gaze palsy, repeated unprovoked falls within 3 years, progressive gait freezing within 3 years, akinetic rigidity, and ideomotor apraxia), supportive clinical cue (poor levodopa response), and supportive imaging finding (midbrain atrophy and bilateral striatal dopaminergic denervation despite asymmetricity).
3 Given the combination of clinical features, the clinical predominance type of our patient was probably PSP with Richardson’s syndrome, with progressive gait freezing or predominant parkinsonism.
3
In our patient, axial rigidity, postural instability/falls, and vertical gaze palsy can be classified as progressive supranuclear palsy syndrome (PSPS) among the five phenotypes of CBD.
2 PSPS accounted for about one-fourth of pathologically confirmed CBD cases.
2 Axial rigidity, gait abnormalities, postural instability, and falls were observed only in about one-third of CBD cases at presentation.
2 Therefore, our patient was more likely to be diagnosed with PSP, not CBD, in the early stages. Cortical dysfunction around 3 years after diagnosis suggested clinical CBD.
2
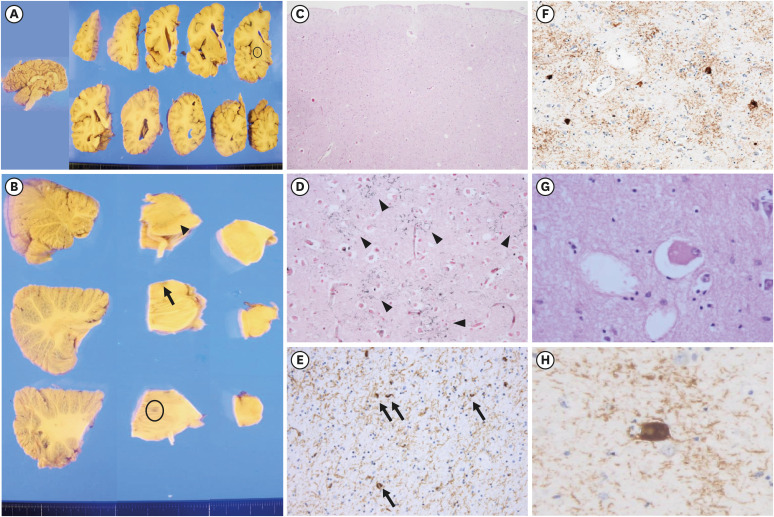
Regarding the macroscopic findings of CBD, the SN was usually hypopigmented, whereas LC pigment may be grossly preserved.
7 Our patient also had less hypopigmentation on the LC; however, these findings were reported in typical PSP.
6 Second, neuronal loss and gliosis of the SN are observed in both PSP and CBD. However, cortical involvement is prominent in CBD, whereas the involvement of BG is prominent in PSP.
67 Third, tau-positive threads in CBD were diffusely distributed in the subcortical WM and gray matter (GM), GP, BG, STN, thalamus, brainstem, and cerebellum.
67 The distribution of tau-immunopositive threads in PSP tends to overlap with that of CBD.
6 The density of tau-positive threads in CBD were equally presented in both GM and WM, or more observed in the WM.
6 Tau-positive threads in both the cortical and subcortical areas in our patient appear to be more relevant to CBD. Fourth, ballooned neurons of the cortical areas in CBD have a diagnostic significance, but they are very rare as the PSP pathology.
6 Our patient had ballooned neurons in the anterior cingulate gyrus and amygdala, and the findings do not carry strong diagnostic significance.
67 Ballooned neurons in the anterior cingulate gyrus and amygdala did not have a diagnostic significance in our patient. Fifth, PSP usually demonstrated symmetrical cortical atrophy, but CBD usually presented with cortical involvement and laterality.
6 Finally, astrocytic plaques affecting the cortices and striatum are significantly pathognomonic findings of CBD.
6 In our patient, astrocytic plaques were observed in the cerebral cortices, consistent with the pathologic diagnosis of CBD.
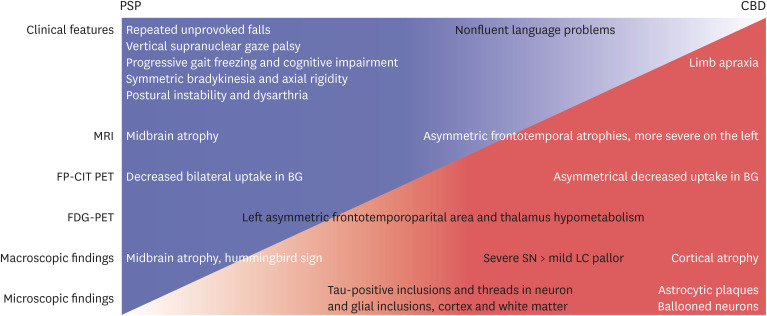
In summary, clinical findings such as supranuclear palsy, symmetric akinesia, rigidity, dysarthria and dysphagia, and midbrain atrophy in MRI and gross examination (hummingbird sign), decreased FP-CIT uptake in both BG more closely resembled PSP (
Fig. 3). On the contrary, cortical dysfunction, asymmetric cortical atrophy in MRI, asymmetric decrease in FP-CIT uptake in the BG, and less severe LC pallor (not always) favor CBD. Microscopically, astrocytic plaques, tau-positive neurons, glia, and threads in the cortex and subcortical areas confirmed the final diagnosis as CBD. Moreover, right cortical infarction, multiple lacunes, and arteriosclerosis in the BG may contribute to the symptoms of our patient. They had probably made the initial diagnosis of CBD difficult.
Typical cases of CBD and PSP can be easy to clinically differentiate, but the definitive diagnosis of PSP and CBD have been only made pathologically. Thus, understanding the complex clinical course of CBD and PSP through a sufficient follow-up period will aid in making a more reliable diagnosis.





 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download