Abstract
Owing to in-hospital transmission of coronavirus disease 2019 (COVID-19), Uijeongbu St. Mary’s Hospital, a university-affiliated hospital in South Korea, was temporarily closed for disinfection in March 2020. This study aimed to investigate the impact of both the hospital shutdown and the prolonged COVID-19 pandemic on short-term outcomes of colorectal cancer (CRC) patients. We retrospectively reviewed the clinicopathologic data of 607 patients who were surgically treated for CRC from May 2018 to September 2021. Nodal upstaging, higher lymphatic invasion and abdominoperineal resection rates for 3 months after the hospital resumed surgery following the shutdown in the first wave of the COVID-19 pandemic were detected, without worse short-term morbidity or mortality. The incidence of adverse pathologic features of CRC such as lymphatic, venous, and perineural invasion was higher throughout the COVID-19 pandemic era. Further follow-up of CRC patients treated in the pandemic era for long-term oncologic outcomes is needed.
Graphical Abstract
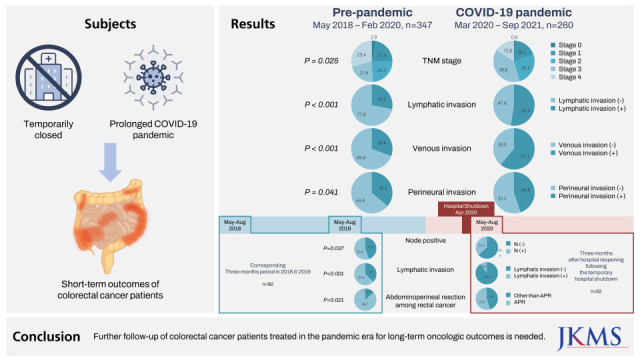
In March 2020, in the middle of the first wave of the coronavirus disease 2019 (COVID-19) pandemic, COVID-19 outbreaks occurred in two university-affiliated hospitals in Seoul and Uijeonbu-si in the northeast region of the metropolis, with epidemiological links between the outbreaks.1 Uijeongbu St. Mary’s Hospital, in Uijeonbu-si, was temporarily closed to disinfect the hospital and quarantine the patients and staff who had contact with patients with COVID-19, and all inpatients and employees were tested for severe acute respiratory syndrome-coronavirus-2 using polymerase chain reaction. The period from hospital shutdown to complete resumption of services was approximately four weeks. The hospital was the only university-affiliated hospital in the northeast area of the metropolis until a new university-affiliated hospital opened in 2021. Therefore, concerns arose about potential delays in the diagnosis and treatment, or disruption of ongoing care of patients with cancer during this period. After resumption of the hospital services for a few months, it appeared that the clinical stages of colorectal cancer (CRC) among newly diagnosed patients were higher, and the symptoms were more severe than those during the corresponding months in previous years. To investigate whether this impression was based upon any evidence, we reviewed the clinicopathologic data of patients who underwent surgical treatment for CRC from May 2018 to September 2021. First, to evaluate the consequences of the temporary hospital shutdown, we compared short-term clinicopathologic outcomes of the patients who were treated during three months after the hospital reopened (May 11 to August 10, 2020) with patients who were treated during the corresponding three-month period in 2018 and 2019. Second, we compared clinicopathologic outcomes of CRC patients during the pre-pandemic (May 2018 to March 2020) and pandemic (April 2020 to September 2021) periods to determine the overall impact of the prolonged COVID-19 pandemic. Patients who underwent ileostomy repair or surgical intervention due to recurrent disease were excluded if they were under regular surveillance. We retrospectively reviewed the medical records of 607 patients with CRC.
All statistical analyses were performed using SPSS version 21 (IBM Corp., Armonk, NY, USA). Continuous variables were analyzed using one-way analysis of variance, and categorical variables were analyzed using the chi-square test or Fisher’s exact test. P values < 0.05 were considered statistically significant.
During the hospital shutdown, 48 CRC patients were in the middle of cycles of chemotherapy (CTx) (18 patients under adjuvant CTx and 30 patients under palliative CTx) and two patients needed elective surgical resection. The two patients who needed surgery were referred to another hospital and underwent surgery elsewhere. Of the patients under CTx, 7 were referred to another hospital for CTx, and one was lost to follow-up. In order to minimize disruption of treatment continuity, a further 40 patients restarted CTx in our institution after a day care center for cancer patients who needed CTx was reopened under the control of an epidemiologist, 10 days after the hospital shutdown started. As a result, 24 patients underwent CTx within 2 weeks after their scheduled date, and 10 patients had a 2–4 week delay. Six patients refused to undergo CTx during the shutdown period and so their CTx schedule was delayed by over 4 weeks.
To assess the impact of the temporary hospital closure, clinicopathological data of two groups (May to August 2018 and May to August 2019, n = 92) vs. after hospital reopening, May to August 2020, n = 52) were compared. After the hospital reopening, the incidence of node metastases (P = 0.037), lymphatic invasion (LI) (P < 0.001), and abdominoperineal resection of rectal cancer (P = 0.021) increased. No statistically significant differences were observed in demographics, cancer location, T stage, M stage, aggressive histology, venous invasion (VI), and perineural invasion (PNI) (Table 1). There were also no significant differences in proportion of Medicare receivers (10 [10.9%] vs. 6 [11.5%]), admission via the emergency department (29 [31.5%] vs. 18 [34.6%]), higher American Society of Anesthesiologists classification (≥ 3) (22 [23.9%] vs. 18 [34.6%]), severe presentation such as ileus and panperitonitis (15 [16.3%] vs. 5 [9.6%]), colonic stent insertion (10 [10.9%] vs. 6 [11.5%]), cancer perforation (7 [7.6%] vs. 3 [5.8%]), emergency surgery (4 [4.3%] vs. 0 [0%]), 30-day morbidity (Clavien-Dindo classification ≥ 3b) (7 [7.6%] vs. 4 [7.7%]), in-hospital mortality (1 [1.1%] vs. 0 [0%]) or length of hospital stay (8.6 ± 7.9 vs. 8.7 ± 7.3 postoperative days). Among patients under the age of 80 years, the proportion who were not compliant with CTx did not increase significantly (11 [13.4%] vs. 9 [23.1%], P = 0.198).
Regarding the overall impact of the prolonged COVID-19 pandemic (comparison between the pre-pandemic, n = 347 vs. pandemic periods, n = 260), the distribution by TNM stage was changed (P = 0.025); in addition, LI (P < 0.001), VI (P < 0.001) and PNI (P = 0.041) rates were significantly higher during the pandemic period (Table 1). Among patients with early CRC (TNM stage ≤ 2), LI, VI and PNI increased markedly during the pandemic period (Fig. 1).
Surprisingly, emergency surgery, severe symptom presentation, perioperative morbidity, and mortality rates did not increase during the 3 months after the hospital reopening, even though the hospital was the only university-affiliated hospital in the northeastern area of the metropolis at the time. This suggests that emergency or urgent cases were referred to hospitals outside the jurisdiction during the shutdown period. Owing to good control of community spread by the testing-tracing-treatment strategy,2 high medical accessibility was maintained during the first wave of the COVID-19 pandemic, and patients were able to use the available medical resources. We observed higher rate of abdominoperineal resection for rectal cancer, nodal upstaging, and more frequent LI after the temporary hospital shutdown period. The prolonged pandemic was related to frequent LI, VI, PNI, and change ofthe distribution by TNM stage.
Recent studies of the effect of the COVID-19 pandemic on CRC have shown that the number of patients with poor prognostic factors has increased. Lim et al.3 reported an increase in LI and the proportion of initially unresectable stage 4 CRC, despite no significant change in the overall proportion of stage 4 CRC. Choi et al.4 reported increased surgical aggressiveness such as a reduced proportion of minimally invasive surgery, increased combined resection rate of adjacent organs and decreased resection potential.
However, the degree of adverse effects of the COVID-19 pandemic on CRC patients seems less marked in Korea than in some other countries that have experienced lockdowns. There have been reports of disease progression and increased mortality in the Philippines,5 and a large decrease in the number of new referrals and elective resection surgeries for CRC in France6 and England.7 Unlike these reports, Lee et al.8 reported no notable changes in cancer care in a study that compared patients diagnosed in 2020 vs in 2019 at Korea University Anam Hospital. In addition, there has been no marked upshifting of stage.34 The lack of a marked change in Korea may be because most medical resources for routine cancer care were not restricted during the pandemic. However, as we noted nodal upstaging in the period after hospital reopening following the shutdown in this study, limited accessibility to any degree can have an adverse effect on the outcome in cancer patients.
Although we did not find nodal upstaging when we analyzed the data throughout the pandemic period, the occurrence of LI, VI and PNI increased. Since they are all independent poor prognostic factors and patients with stage 2 CRC need to undergo adjuvant chemotherapy if any of these high-risk features are present,910 attention should be paid to the prognosis of CRC patients treated during the pandemic. Recently, there has been a profound resurgence of COVID-19 during the fifth wave caused by the Omicron variant. This might have a negative effect on CRC management due to delay of surgery caused by absenteeism among surgeons and other medical staffs with infection leading to reduced admission capacity in individual hospitals, resulting in a decrease in new referrals, elective surgeries, and screening procedures for CRC along with increased number of CRC patients with infection. Further examination of trends in CRC stage, referral rates for cancer screening and treatment, and long-term oncologic outcomes of patients diagnosed and treated during the pandemic era is required
This study has limitations due to its retrospective nature in a single institution.
In conclusion, we found nodal upstaging, higher LI and abdominoperineal resection rate after the hospital reopening following a shutdown during the first wave of the COVID-19 pandemic, without worse short-term morbidity or mortality. We also observed an increased occurrence of adverse pathologic features of CRC such as LI, VI and PNI during the COVID-19 pandemic era. Further follow-up for long-term oncologic outcomes in patients diagnosed with CRC during the pandemic is essential.
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Uijeongbu St. Mary’s Hospital, The Catholic University of Korea, South Korea (approval No. UC20RISI0127). The Institutional Review Board waived the requirement for obtaining informed consent from patients.
References
1. Park K, Lee J, Lee K, Jung J, Kim SH, Lee J, et al. Epidemiologic linkage of COVID-19 outbreaks at two university-affiliated hospitals in the Seoul Metropolitan Area in March 2020. J Korean Med Sci. 2021; 36(4):e38. PMID: 33496089.
2. Park Y, Huh IS, Lee J, Kang CR, Cho SI, Ham HJ, et al. Application of testing-tracing-treatment strategy in response to the COVID-19 outbreak in Seoul, Korea. J Korean Med Sci. 2020; 35(45):e396. PMID: 33230987.
3. Lim JH, Lee WY, Yun SH, Kim HC, Cho YB, Huh JW, et al. Has the COVID-19 pandemic caused upshifting in colorectal cancer stage? Ann Coloproctol. 2021; 37(4):253–258. PMID: 34376026.
4. Choi JY, Park IJ, Lee HG, Cho E, Kim YI, Kim CW, et al. Impact of the COVID-19 pandemic on surgical treatment patterns for colorectal cancer in a tertiary medical facility in Korea. Cancers (Basel). 2021; 13(9):2221. PMID: 34066390.
5. Manlubatan SI, Lopez MP, Onglao MA, Monroy Iii HJ. Modifications to treatment plan of rectal cancer in response to COVID-19 at the Philippine General Hospital. Ann Coloproctol. 2021; 37(4):225–231. PMID: 34364319.
6. Priou S, Lamé G, Chatellier G, Tournigand C, Kempf E. Effect of the COVID-19 pandemic on colorectal cancer care in France. Lancet Gastroenterol Hepatol. 2021; 6(5):342–343.
7. Morris EJ, Goldacre R, Spata E, Mafham M, Finan PJ, Shelton J, et al. Impact of the COVID-19 pandemic on the detection and management of colorectal cancer in England: a population-based study. Lancet Gastroenterol Hepatol. 2021; 6(3):199–208. PMID: 33453763.
8. Lee S, Lim AR, Kim MJ, Choi YJ, Kim JW, Park KH, et al. Innovative countermeasures can maintain cancer care continuity during the coronavirus disease-2019 pandemic in Korea. Eur J Cancer. 2020; 136:69–75. PMID: 32652443.
9. Liebig C, Ayala G, Wilks J, Verstovsek G, Liu H, Agarwal N, et al. Perineural invasion is an independent predictor of outcome in colorectal cancer. J Clin Oncol. 2009; 27(31):5131–5137. PMID: 19738119.
10. Mollberg NM, Bennette C, Howell E, Backhus L, Devine B, Ferguson MK. Lymphovascular invasion as a prognostic indicator in stage I non-small cell lung cancer: a systematic review and meta-analysis. Ann Thorac Surg. 2014; 97(3):965–971. PMID: 24424014.
Fig. 1
Comparison of proportion of lymphatic, venous and perineural invasion in patients with colorectal cancer according to TNM stage during the pre-pandemic (May 2018–February 2020) vs. the coronavirus disease 2019 pandemic (March 2020–September 2021).

Table 1
Impact of the temporary hospital shutdown during the 1st wave of the COVID-19 pandemic and the prolonged COVID-19 pandemic on short-term clinicopathologic outcomes of patients with colorectal cancer
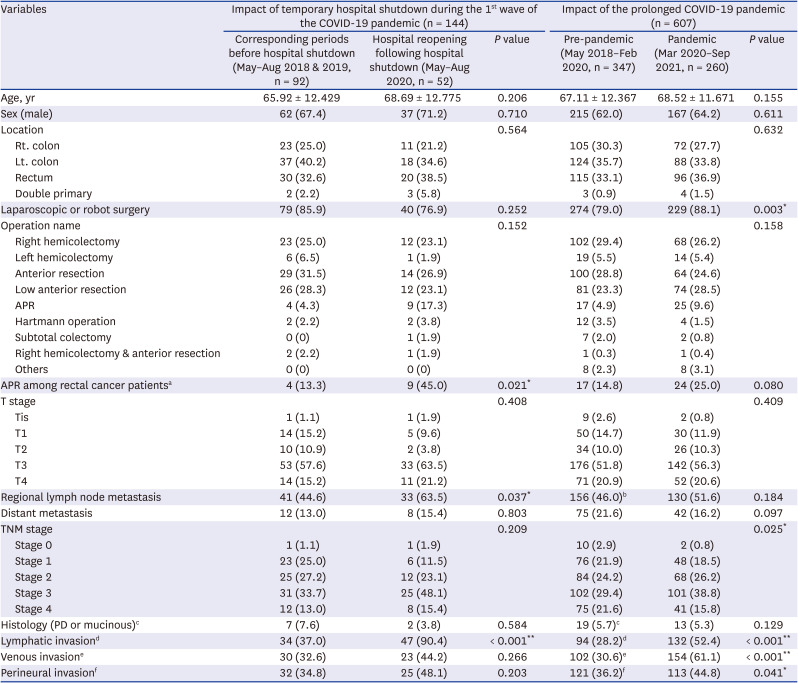
Data are presented as number (%) or mean ± standard deviation.
COVID-19 = coronavirus disease 2019, Rt. = right, Lt. = left, APR = abdominoperineal resection, PD = poorly-differentiated.
aThe number of the patients with rectal cancer in each group were 30 vs. 20 and 115 vs. 96; bRegional node metastasis status was not available for 8 patients in the pre-pandemic group and 8 patients in the pandemic group; cLymphatic invasion status was not available for 14 patients in the pre-pandemic group and 8 patients in the pandemic group; dVenous invasion status was not available for 14 patients in the pre-pandemic group and 8 patients in the pandemic group; fPerineural invasion status was not available for 13 patients in the pre-pandemic group and 8 patients in the pandemic group.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download