Abstract
Despite the accuracy of nucleic acid amplification tests (NAATs), rapid antigen tests (RATs) for severe acute respiratory syndrome coronavirus-2 are widely used as point-of-care tests. A total of 282 pairs of reverse transcription-polymerase chain reaction and Standard Q COVID-19 Ag tests were serially conducted for 68 patients every 3–4 days until their discharge. Through a field evaluation of RATs using direct nasopharyngeal swabs, the sensitivities were 84.6% and 87.3% for E and RNA-dependent RNA polymerase (RdRp) genes, respectively, for specimens with cycle thresholds (Cts) < 25. The Ct values of E and RdRp genes for 95% detection rates by RATs were 16.9 and 18.1, respectively. The sensitivity of RAT was 48.4% after the onset of symptoms, which was not sufficient. RAT positivity gradually decreased with increased time after symptom onset and had continuously lower sensitivity than NAATs.
Graphical Abstract
Nucleic acid amplification tests (NAATs) of the pathogen that causes coronavirus disease 2019 (COVID-19), severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), is the diagnostic reference standard for COVID-19.12 However, the drawbacks of conventional molecular testing include high cost, longer turnaround times of about 3–4 hours, and the requirement for specialized instruments and skilled laboratory personnel. In addition, NAAT results can remain positive for weeks to months after an initial infection and can detect viral nucleic acids, even when the virus cannot be cultured.3 To counter these drawbacks, several rapid antigen tests (RATs) for SARS-CoV-2 were developed and used as point-of-care tests. Most RATs are lateral-flow immunochromatographic assays using nasopharyngeal (NP) swab specimens and give results in less than 30 minutes.45 Despite their lower sensitivity compared with molecular testing, they are widely used for the rapid diagnosis of COVID-19 and prevention of SARS-CoV-2 transmission.
The Standard Q COVID-19 Ag kit (SD Biosensor, Suwon, Korea) is currently one of the most widely used RATs for COVID-19 detection in the Republic of Korea, and its clinical performance has been evaluated in several studies.67891011121314 However, those studies mainly focused on the diagnostic accuracy of the RAT. Besides, there were few data about the duration of positive RAT results after initial confirmation of COVID-19 and whether subsequent negative RAT results were sufficient to release a patient from isolation. In the present study, the evaluation of the Standard Q COVID-19 Ag kit was conducted with direct NP specimens from symptomatic COVID-19 patients and serially followed up with NAATs for the duration of their hospital stay.
The present study was conducted as a prospective study. We enrolled patients who displayed COVID-19 symptoms within 2 days who had been admitted to the Hyundae General Hospital affiliated with the Chung-Ang University Health Care System from December 20, 2020 to March 12, 2021. The following data were acquired: sex, height, weight, birthdate, admission and discharge date, symptoms, past medical history of underlying diseases, and fever duration. When admitted, we classified the severity of the patients’ symptoms using the ordinal scale (1: no limitation of activities, 2: limitation of activities, 3: oxygen by nasal prongs, 4: oxygen by facial mask, 5: non-invasive ventilation, and 6: mechanical ventilation).
For laboratory diagnosis of COVID-19, 282 pairs of NAATs and RATs were serially conducted on 68 patients every 3–4 days until their discharge. For NAATs, real-time reverse transcriptase-polymerase chain reaction (rRT-PCR) was conducted on the extracted RNA with the Real-Q 2019-nCoV Detection Kit (Biosewoom, Seoul, Korea) using a CFX96 Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). The targets were the E and RNA-dependent RNA polymerase (RdRp) genes. A sample was considered “SARS-CoV-2-positive” when the cycle threshold (Ct) values of all genes were ≤ 38. If only one of the genes had a Ct ≤ 38, the test results were interpreted as “inconclusive.”1 A sample was considered “negative” when the Ct values of all the genes were > 38. RATs were conducted using direct NP swab specimens and performed according to the manufacturer’s guidelines. This RAT targeted the nucleocapsid protein of SARS-CoV-2.
Diagnostic sensitivity and specificity were calculated and compared with those of the NAAT results according to the numbers of followed-up days after the patients’ admissions. We additionally performed binomial logistic regression analysis for evaluation of the clinical performance of RATs. Logistic regression models were constructed based on the binomial results of the RATs and the Cts of each 2 target genes (E and RdRp) included in the RT-PCR assay. This analysis allowed us to predict the relationship between the RAT results and the Ct value of each target gene and estimate the Ct values for which 95% detection rates were achieved (Ct95). Statistical analyses were performed using R version 4.3.3 (R Foundation for Statistical Computing, Vienna, Austria).
During the study period, 68 patients were enrolled, and 26 (38.2%) were female. The median age was 68.0 (interquartile range, 54.3–81.3) years. The mean hospitalization duration was 12.6 ± 7.4 days, and 51 (75.0%) patients had ≥ 1 underlying diseases (Supplementary Table 1). The most common symptom was fever (72.1%) with a duration of 6.7 ± 4.2 days. The next most common symptom was dyspnea (38.2%), followed by cough (33.8%), sputum production (25.5%), and myalgia (23.5%). The most common initial ordinal scale score was 1 (36.8%), followed by 3 (32.4%) and 5 (16.2%) (Supplementary Table 2).
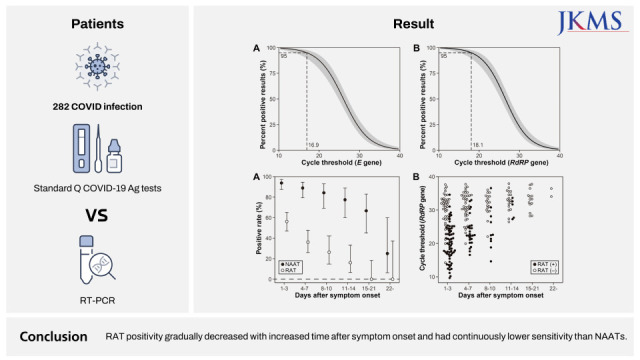
The diagnostic sensitivity of the RAT according to the Ct values for each gene detected in the specimens are listed in Supplementary Table 3. Based on the E gene, the sensitivities of RAT with Ct values < 20, 20–24, 25–29, 30–34, and 35–38 were 91.1%, 78.3, 39.1%, 10.0%, and 5.0%, respectively. For the RdRp gene, the sensitivities of RAT were 92.7%, 82.6%, 37.8%, 12.8%, and 3.7%, respectively. The diagnostic specificity for specimens giving negative or indeterminate PCR results was 100% (95% confidence interval, 95.2–100). Binomial logistic regression analysis revealed that the Ct95 values of the RAT for the E and RdRp genes were 16.9 and 18.1, respectively (Fig. 1).
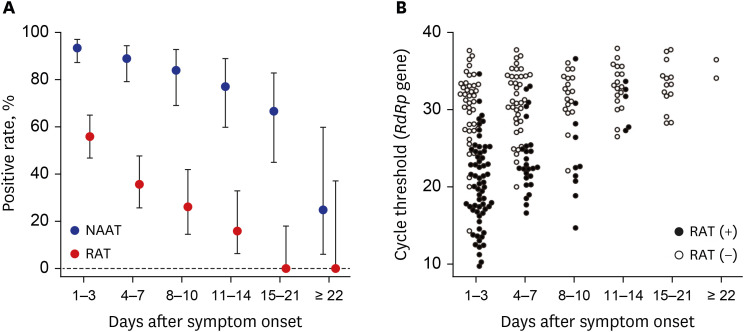
The positivity rate of the RAT for serial samples obtained during the follow-up period, and RAT results for each sample according to the number of days after symptom onset and Ct values are illustrated in Fig. 2. Within 1 to 3 days of symptom onset, the sensitivity of the RAT was 56.3% (63/112); after that, it gradually decreased to 36.1% (4–7 days, 26/72), 26.3% (8–10 days, 10/38), 16.1% (11–14 days, 5/31), and 0% (after 15 days, 0/29). NAATs were generally more sensitive than RATs, with sensitivities of 93.8% (1–3 days, 105/112), 88.9% (4–7 days, 64/72), 84.2% (8–10 days, 32/38), 77.4% (11–14 days, 24/31), 66.7% (15–21 days, 14/21), and 25.0% (after 22 days, 2/8).
Despite the ease, rapidity, and cost-effectiveness of RATs for virus detection, concerns about its low sensitivity and generation of false negative results, especially for specimens with low viral loads, remain. Moreover, several studies have reported a wide range of overall RAT diagnostic sensitivities from 17.5–89.2%.6891011121314 In this context, we evaluated the diagnostic accuracy of the Standard Q COVID-19 Ag kit with direct NP specimens. For specimens with Ct < 25, the sensitivities were 84.6% (77/91 for the E gene) and 87.3% (76/87 for the RdRp gene). Studies focusing on the diagnostic sensitivity of the Standard Q COVID-19 Ag kit generated various results. Korenkov et al.10 evaluated 2,028 NP specimens and reported that the sensitivity for specimens with Ct < 25 was 98.25%. Peña-Rodríguez et al.13 reported that the value was 88%, and Amer et al.9 reported that it was > 96.5%. Among the studies, Oh et al.6 reported the lowest sensitivity at 41.1%. For lower viral-load specimens, our study found a sensitivity lower than or equal to those of previous studies. The sensitivities for specimens with 30 ≤ Ct < 38 were 9% (9/100 for the E gene) and 10.5% (11/105 for the RdRp gene) in this study. Previous studies reported the following values for specimens with 30 ≤ Ct < 38: 65.0%,9 63.6%,8 45.3%,11 and < 10%.13 Binomial logistic regression analysis revealed that the Ct95 values of the Standard Q COVID-19 Ag kit were 16.9–18.1, which were lower than those determined in a previous study.10 This could be a result of the lower sensitivity of RAT for specimens with Ct < 20 compared with previous studies; there were considerable false negative results (7.3–8.9%) among the specimens in this study. Differences in RAT results between studies may be due to patient characteristics, such as the presence of symptoms and/or analytical variations in the testing procedures. Because the testing procedures are performed manually from the NP swab collection to the results readout, RATs conducted using lateral-flow immunochromatographic assays are affected by several analytical variations. Specifically, the NP swabs should be well mixed with the extraction buffer, and drops of the buffer-mixed specimen should be applied; these procedures are dependent on the skill level of the technician.
In the analysis of the positive rate according to the number of days after symptom onset, the positive rates of RATs were also lower than those determined by NAATs. Even within 7 days of symptom onset, the sensitivity was only 48.4% (89/184), indicating that RAT generated a false-negative rate of 51.6% in symptomatic COVID-19 patients. Thus, RATs alone should not be used to rule out COVID-19, even in the early period after symptom onset. RATs not only perform poorly for diagnosis but also for determining the release of patients from isolation. Previous studies demonstrated that virus detected with Ct values lower than 30 and/or of samples obtained after 10 days can be contagious or culturable.1516 In this study, there were 6 patients with viral loads of Ct < 30 (RdRp gene) whose samples were obtained 10 days after symptom onset. Among them, 2 patients (33.3%) were negative according to the RAT. Although we did not assess the viral cultures of the specimens from those patients simultaneously, those samples could have had a residual risk of viral transmission.17
In conclusion, the diagnostic sensitivity of RAT was unsatisfactory, even for specimens with high viral loads or in the initial period after symptom onset. In addition, RAT negativity is not likely to guarantee non-transmission of the virus. Clinicians should always be aware of the possibility of false negative results of RATs.
References
1. Hong KH, Lee SW, Kim TS, Huh HJ, Lee J, Kim SY, et al. Guidelines for laboratory diagnosis of coronavirus disease 2019 (COVID-19) in Korea. Ann Lab Med. 2020; 40(5):351–360. PMID: 32237288.
2. Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020; 25(3):2000045.
3. Kim MC, Cui C, Shin KR, Bae JY, Kweon OJ, Lee MK, et al. Duration of culturable SARS-CoV-2 in hospitalized patients with COVID-19. N Engl J Med. 2021; 384(7):671–673. PMID: 33503337.
4. Somborac Bačura A, Dorotić M, Grošić L, Džimbeg M, Dodig S. Current status of the lateral flow immunoassay for the detection of SARS-CoV-2 in nasopharyngeal swabs. Biochem Med (Zagreb). 2021; 31(2):020601. PMID: 34140830.
5. Surasi K, Cummings KJ, Hanson C, Morris MK, Salas M, Seftel D, et al. Effectiveness of Abbott BinaxNOW Rapid Antigen Test for detection of SARS-CoV-2 infections in outbreak among horse racetrack workers, California, USA. Emerg Infect Dis. 2021; 27(11):2761–2767. PMID: 34469287.
6. Oh SM, Jeong H, Chang E, Choe PG, Kang CK, Park WB, et al. Clinical application of the Standard Q COVID-19 Ag test for the detection of SARS-CoV-2 infection. J Korean Med Sci. 2021; 36(14):e101. PMID: 33847084.
7. Chaimayo C, Kaewnaphan B, Tanlieng N, Athipanyasilp N, Sirijatuphat R, Chayakulkeeree M, et al. Rapid SARS-CoV-2 antigen detection assay in comparison with real-time RT-PCR assay for laboratory diagnosis of COVID-19 in Thailand. Virol J. 2020; 17(1):177. PMID: 33187528.
8. Kim HW, Park M, Lee JH. Clinical evaluation of the rapid STANDARD Q COVID-19 Ag test for the screening of severe acute respiratory syndrome coronavirus 2. Ann Lab Med. 2022; 42(1):100–104. PMID: 34374355.
9. Amer RM, Samir M, Gaber OA, El-Deeb NA, Abdelmoaty AA, Ahmed AA, et al. Diagnostic performance of rapid antigen test for COVID-19 and the effect of viral load, sampling time, subject’s clinical and laboratory parameters on test accuracy. J Infect Public Health. 2021; 14(10):1446–1453. PMID: 34175237.
10. Korenkov M, Poopalasingam N, Madler M, Vanshylla K, Eggeling R, Wirtz M, et al. Evaluation of a rapid antigen test to detect SARS-CoV-2 infection and identify potentially infectious individuals. J Clin Microbiol. 2021; 59(9):e0089621. PMID: 34213977.
11. Nalumansi A, Lutalo T, Kayiwa J, Watera C, Balinandi S, Kiconco J, et al. Field evaluation of the performance of a SARS-CoV-2 antigen rapid diagnostic test in Uganda using nasopharyngeal samples. Int J Infect Dis. 2021; 104:282–286. PMID: 33130198.
12. Ristić M, Nikolić N, Čabarkapa V, Turkulov V, Petrović V. Validation of the STANDARD Q COVID-19 antigen test in Vojvodina, Serbia. PLoS One. 2021; 16(2):e0247606. PMID: 33617597.
13. Peña-Rodríguez M, Viera-Segura O, García-Chagollán M, Zepeda-Nuño JS, Muñoz-Valle JF, Mora-Mora J, et al. Performance evaluation of a lateral flow assay for nasopharyngeal antigen detection for SARS-CoV-2 diagnosis. J Clin Lab Anal. 2021; 35(5):e23745. PMID: 33675086.
14. Berger A, Nsoga MT, Perez-Rodriguez FJ, Aad YA, Sattonnet-Roche P, Gayet-Ageron A, et al. Diagnostic accuracy of two commercial SARS-CoV-2 antigen-detecting rapid tests at the point of care in community-based testing centers. PLoS One. 2021; 16(3):e0248921. PMID: 33788882.
15. Routsias JG, Mavrouli M, Tsoplou P, Dioikitopoulou K, Tsakris A. Diagnostic performance of rapid antigen tests (RATs) for SARS-CoV-2 and their efficacy in monitoring the infectiousness of COVID-19 patients. Sci Rep. 2021; 11(1):22863. PMID: 34819567.
16. Zhou J, Otter JA, Price JR, Cimpeanu C, Meno Garcia D, Kinross J, et al. Investigating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) surface and air contamination in an acute healthcare setting during the peak of the coronavirus disease 2019 (COVID-19) pandemic in London. Clin Infect Dis. 2021; 73(7):e1870–e1877. PMID: 32634826.
17. Kohmer N, Toptan T, Pallas C, Karaca O, Pfeiffer A, Westhaus S, et al. The comparative clinical performance of four SARS-CoV-2 rapid antigen tests and their correlation to infectivity in vitro. J Clin Med. 2021; 10(2):328. PMID: 33477365.
SUPPLEMENTARY MATERIALS
Supplementary Table 1
Demographics of enrolled coronavirus disease 2019 patients
Supplementary Table 2
Clinical and radiological manifestations of COVID-19 in patients
Supplementary Table 3
Diagnostic sensitivity of the Standard Q COVID-19 Ag kit, the rapid point-of-care severe acute respiratory syndrome coronavirus-2 antigen assay, compared with real-time reverse transcriptase-polymerase chain reaction assays
Fig. 1
Logistic regression analyses for the percentage of positive results from the Standard Q COVID-19 Ag kit according to the cycle thresholds of E (A) and RdRp (B) genes in the severe acute respiratory syndrome coronavirus-2 reverse transcriptase-polymerase chain reaction.

Fig. 2
(A) Positive rates of the Standard Q COVID-19 Ag kit and nuclear acid amplification tests according to the number of days after symptom onset. (B) RAT (Standard Q COVID-19 Ag kit) results of COVID-19 patients according to the number of days after symptom onset and cycle threshold values. Error bars represent 95% confidence intervals.
NAAT = nucleic acid amplification test, RAT = rapid antigen test.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download