This article has been
cited by other articles in ScienceCentral.
Abstract
Croup is an acute upper respiratory disease primarily caused by the parainfluenza virus. Owing to inflammation and edema of the upper airways, children present with barky cough and stridor, and some may experience respiratory distress. We investigated children aged < 5 years with coronavirus disease 2019 (COVID-19) admitted to two hospitals in Seoul, South Korea, and observed a spike in croup cases during the omicron surge. Among the 569 children admitted from March 1, 2021 to February 25, 2022, 21 children (3.7%) had croup, and the proportion of croup cases was significantly higher during the omicron wave than that during the delta wave (12.4% vs. 1.2%, P < 0.001). With the immediate administration of corticosteroids and epinephrine via nebulizer, the symptoms improved rapidly. During the current omicron surge, careful monitoring of the symptoms of croup in young children is needed for the diagnosis of COVID-19 and its timely management.
Go to :

Graphical Abstract
Go to :

Keywords: Croup, Children, SARS-CoV-2, COVID-19, Omicron
Croup (acute laryngotracheitis and laryngotracheobronchitis) is a common childhood upper respiratory disease characterized by the abrupt onset of barky cough accompanied by stridor, a hoarse voice, and respiratory distress.
1 These symptoms result from upper airway inflammation, edema, and, ultimately, obstruction caused by acute respiratory viral infections, most typically parainfluenza types 1, 2 and 3.
2 Sporadic case reports suggest severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as a pathogen responsible for croup, and many clinicians have observed spikes in croup cases during the current omicron (B.1.1.529) wave.
345678910 Herein, we report a recent sharp rise in pediatric coronavirus disease 2019 (COVID-19) cases requiring hospitalization due to croup during the omicron surge in Seoul, South Korea.
The present study analyzed young children aged < 5 years diagnosed with COVID-19 and hospitalized in the Seoul Metropolitan Government-Seoul National University Boramae Medical Center (SMG-SNU BMC) and National Medical Center (NMC) from March 1, 2021, to February 25, 2022. These hospitals actively responded to patients with COVID-19 and accommodated young children with COVID-19 in Seoul. The medical records of the patients were retrospectively reviewed, and data on symptoms, signs, laboratory examinations, radiologic examinations, medications, and clinical course were collected. Croup was diagnosed when the patient had a barky cough and stridor. The severity of croup was classified as mild, moderate, and severe based on the Westley croup score, which has been most extensively studied.
11 Mild croup was defined by Westley croup scores ≤ 2, that is, when there was occasional stridor with agitation, and mild or no chest retractions. Moderate croup was defined by Westley croup scores of 3–7, when the child had stridor at rest and mild to moderate retractions but had no distress. Severe croup was defined by Westley croup scores of 8–11, when the child had stridor at rest, marked chest retractions, and significant distress and agitation. Monthly data on confirmed cases of COVID-19 in children aged < 5 years in Seoul were collected from the Seoul Metropolitan Government database, and the monthly detection rate of respiratory virus in South Korea was collected using publicly available data from the Korea Disease Control and Prevention Agency (KDCA). The proportion of croup cases during the omicron wave (January to February 2022) was compared with that during the delta wave (July to December 2021) using the Pearson χ
2 test.
From March 1, 2021, to February 25, 2022, 569 children aged < 5 years with COVID-19 were hospitalized. Of them, 21 (3.7%) children had croup, of which 17 (81.0%) of the cases occurred in the last two months when the omicron variant was dominant in Korea and COVID-19 cases in children aged < 5 years surged in Seoul (
Fig. 1A). The proportion of croup cases was significantly higher during the omicron wave (12.4%) than that during the delta wave (1.2%,
P < 0.001). The parainfluenza virus did not circulate between January and February 2022.
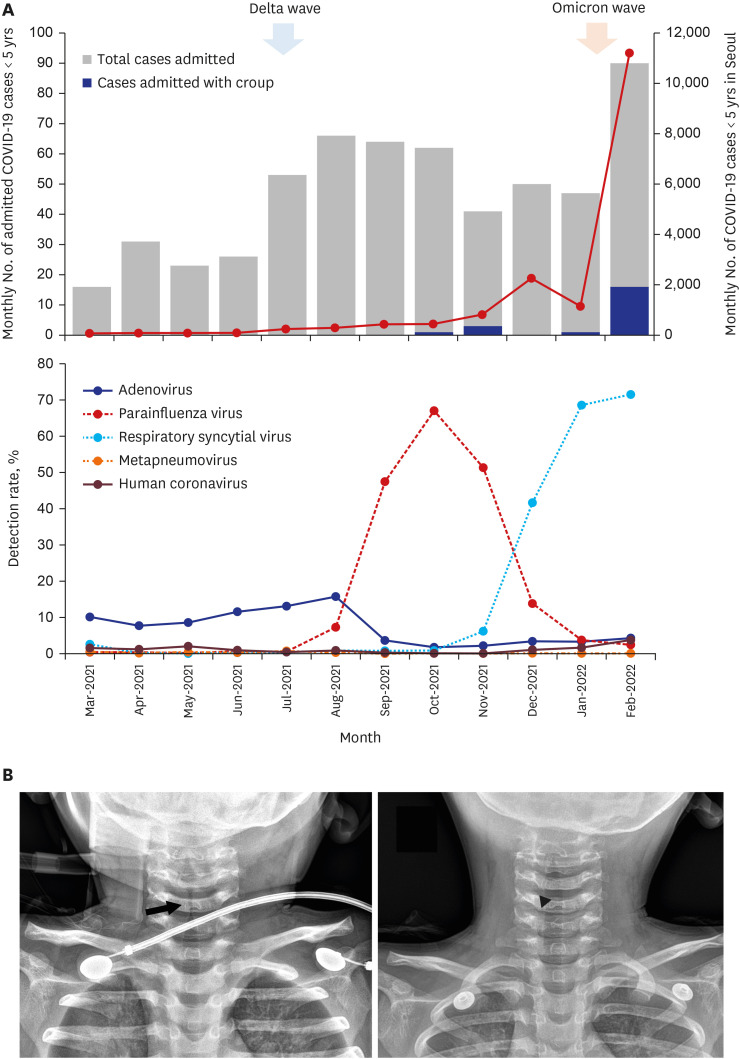 | Fig. 1
Croup due to SARS-CoV-2 infection in children < 5 years. (A) Monthly cases of COVID-19 in children < 5 years and monthly detection rate of respiratory viruses. With the sharp rise in the number of COVID-19 cases in Seoul, South Korea during the omicron wave, the proportion of croup cases admitted to two hospitals in Seoul significantly increased. The parainfluenza virus did not circulate in South Korea between January and February 2022. (B) Neck radiography of a 28-month-old child with COVID-19 presenting with severe croup. Subglottic narrowing is noted (arrow, left) and with medical treatment the airway became patent after 18 hours (arrowhead, right).
COVID-19 = coronavirus disease 2019.

|
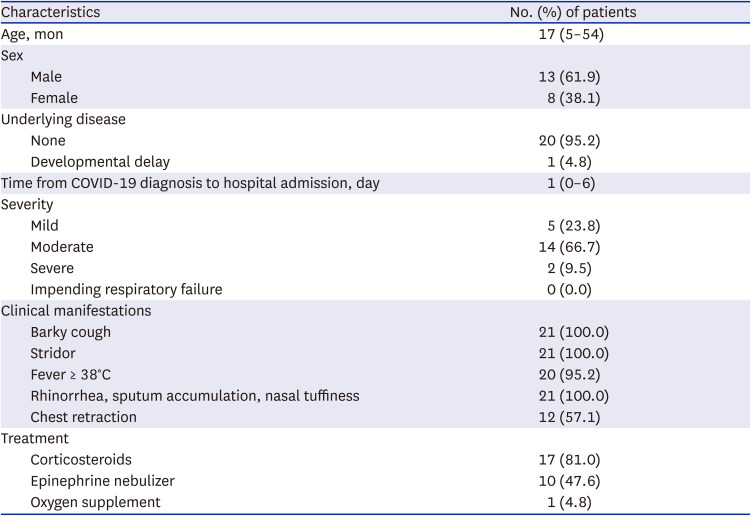
The median age of the 21 patients was 17 months (range, 5–54 months), and 61.9% of the patients were male children (
Table 1). The median time from COVID-19 diagnosis to hospital admission was 1 day (range, 0–6 days). Five (23.8%) patients had mild croup; 14 (66.7%), moderate croup; and, 2 (9.5%), severe croup. Most of the children with croup presented with fever along with barky cough and stridor, and other upper respiratory symptoms including rhinorrhea, sputum accumulation, and nasal stuffiness. Laboratory tests were performed on 15 (71.4%) children, and the results were unremarkable. A respiratory virus panel test was performed in one child, and it was negative for parainfluenza virus, influenza virus, respiratory syncytial virus, human metapneumovirus, and rhinovirus. Twelve (57.1%) patients had chest retraction during the physical examinations. Eighteen (85.7%) children required immediate administrations of corticosteroids, epinephrine nebulizers, or both, to relieve their symptoms. Corticosteroids were administered to 17 children (81.0%) (
Table 1). Dexamethasone (0.6 mg/kg, via intramuscular, intravenous, or per os route) was administered once in 14 (82.4%) children, oral prednisolone (1 mg/kg) was administered once in one (5.9%) child, and intravenous dexamethasone (0.15 mg/kg) was administered in one child twice with an interval of 12 hours. One child with severe croup received 0.6 mg/kg of intravenous dexamethasone twice with an interval of 7 hours. Epinephrine nebulizer was administered to 10 (47.6%) children, and 70% of the children received it more than three times.
Table 1
Clinical characteristics of 21 children with COVID-19 hospitalized for croup
|
Characteristics |
No. (%) of patients |
|
Age, mon |
17 (5–54) |
|
Sex |
|
|
Male |
13 (61.9) |
|
Female |
8 (38.1) |
|
Underlying disease |
|
|
None |
20 (95.2) |
|
Developmental delay |
1 (4.8) |
|
Time from COVID-19 diagnosis to hospital admission, day |
1 (0–6) |
|
Severity |
|
|
Mild |
5 (23.8) |
|
Moderate |
14 (66.7) |
|
Severe |
2 (9.5) |
|
Impending respiratory failure |
0 (0.0) |
|
Clinical manifestations |
|
|
Barky cough |
21 (100.0) |
|
Stridor |
21 (100.0) |
|
Fever ≥ 38°C |
20 (95.2) |
|
Rhinorrhea, sputum accumulation, nasal tuffiness |
21 (100.0) |
|
Chest retraction |
12 (57.1) |
|
Treatment |
|
|
Corticosteroids |
17 (81.0) |
|
Epinephrine nebulizer |
10 (47.6) |
|
Oxygen supplement |
1 (4.8) |

One 28-month-old child with severe croup required oxygen therapy because of desaturation. The child initially presented with febrile status epilepticus, and seizures were controlled with fosphenytoin administration; additionally, marked stridor with severe chest retraction was noticed. The patient had a high fever of up to 39.7°C and tachycardia, and limited aeration was observed on chest auscultation. Oxygen was supplemented via a facial mask due to desaturation, and steeple appearance was observed on neck radiography (
Fig. 1B). Dexamethasone was administered twice at an interval of 7 hours along with an epinephrine nebulizer due to persistent respiratory distress. The patient then showed gradual improvement, and neck radiography performed 18 hours later showed no stenosis in the upper airway. The presence of high fever and absence of signs of lower respiratory tract infection may preclude the possibility of co-infection with respiratory syncytial virus, which was also prevalent during the last two months.
Croup is caused by acute respiratory viral infections, most typically parainfluenza virus which comprise approximately 39.7–41.8% of pathogen-identified croup.
21213 Other respiratory viruses also responsible for croup include influenza A virus, endemic coronavirus, and respiratory syncytial virus, which comprise approximately 6.5–13.7%, 2.1–17%, and 8.2–15.0% of pathogen-identified croup, respectively.
121314 Croup generally peaks in spring and autumn, reflecting the seasonality of parainfluenza virus.
121315
Sporadic case reports published before the omicron wave suggested a possible association of croup with SARS-CoV-2 in children.
456716 Moreover, we have observed a few cases of croup in children with COVID-19 before the omicron surge in Korea. However, a sharp increase in the number of children presenting with croup symptoms became evident during the omicron surge. Two recent studies conducted in Seattle Children’s Hospital and Boston Children’s Hospital observed a marked increase in cases of croup during the omicron surge.
310 Tests for COVID-19 were more likely to be positive in children with croup during the omicron wave compared to those during the delta wave. Similarly, our study observed a sudden increase in the proportion of children with COVID-19 hospitalized due to moderate to severe croup in February 2022, when the omicron variant became dominant in South Korea.
This notable increase in children with SARS-CoV-2 presenting with croup during the omicron surge might be due to the characteristics of the variant. Recent studies have shown that omicron variants replicate more readily in the upper respiratory tract than in the lower respiratory tract.
1718 The high affinity of the variant to angiotensin-converting enzyme 2 and the usage of the endosomal pathway for cell entry might account for these distinct characteristics of omicron as compared to previously documented variants.
19 Although this finding might be reassuring for adults, children have smaller airway calibers, and severe inflammation of the upper respiratory tract may lead to respiratory difficulty.
Fortunately, croup is a respiratory disease that is familiar to pediatricians and clinicians in emergency departments. With timely diagnosis and appropriate treatment, symptoms of croup generally subside rapidly, and complications are rare. With appropriate management, less than 3% of children with croup require intubation, and no children received mechanical ventilation in our study.
20 Minimal intervention is recommended to avoid agitation that could worsen symptoms in children with croup, and oxygen therapy can be beneficial in children showing signs of respiratory distress.
1 The administration of corticosteroids, usually a single dose of dexamethasone at 0.6 mg/kg (maximum dose 16 mg), is beneficial irrespective of severity. This dose differs from that used for children with severe or critical COVID-19 (0.15 mg/kg/dose once daily for up to 10 days).
21 Treatment with epinephrine via nebulizer effectively reduces the clinical symptoms in children with moderate to severe croup. Meanwhile, the requirement of multiple administration of dexamethasone and nebulized epinephrine observed in our study may imply that croup due to SARS-CoV-2 may be more severe in manifestation compared to other known pathogens.
110
The limitation of our study was that data was retrospectively analyzed and the study was conducted in only two centers, hence the results of our study may not be generalized. Moreover, only one patient underwent a respiratory virus panel test, and coinfections with other respiratory viruses, especially respiratory syncytial virus which was also prevalent in the last two months, were not assessed in the rest of the patients. More than one respiratory virus can be co-detected in approximately 11.0–21.6% of croup cases.
1314 However, previously reported cases and recent studies have demonstrated that SARS-CoV-2 alone could cause croup in children, and the results of our study are valuable to increase the awareness about croup in young children with COVID-19.
471016
In conclusion, with the increase in omicron variant infections that affect the upper respiratory tract, there was a sudden increase in young children with COVID-19 requiring hospitalization due to moderate to severe croup in Seoul. Appropriate guidance on when to seek acute care should be provided for young children with COVID-19 presenting with a barky cough and stridor isolated at home or at a residential treatment center. Healthcare professionals should suspect COVID-19 in young children presenting with symptoms and signs of croup during the omicron wave and provide appropriate treatment along with isolation measures.
Ethics statement
This study was approved by the Institutional Review Board (IRB) of SMG-SNU BMC (IRB No. 30-2020-324) and NMC (IRB No. 2022-0001-002) and informed consent was waived.
Go to :







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download