Abstract
Purpose
Rectal prolapse is hypothesized to be caused due to weakness of the pelvic floor which is related to childbearing. However, half of the female patients with rectal prolapse were reported to be nulliparous and this hypothesis doesn’t explain the prolapse in males. The aim of this study is to evaluate the role of rectal redundancy in rectal prolapse pathophysiology.
Methods
This study was conducted prospectively. Fourteen patients who underwent rectopexy were included in the study group. A total of 17 patients who underwent laparotomy for another reason and who have no symptoms regarding rectal prolapse were included in the control group. In order to measure the redundancy of the rectum, we have calculated the ratio of length of intraperitoneal rectum (R) to length of distance between promontorium and peritoneal reflection (PRx). The primary outcome of this study was to evaluate whether the R/PRx ratio is higher in patients with rectal prolapse compared to the control group.
Results
Comparing the anatomic features showed that the length of sigmoid colon and length of PRx were not significantly different between the two groups. However, the length of intraperitoneal rectum was significantly higher in the prolapse group. Furthermore, the median R/PRx ratio in the prolapse group was significantly higher than in the control group.
Go to : 
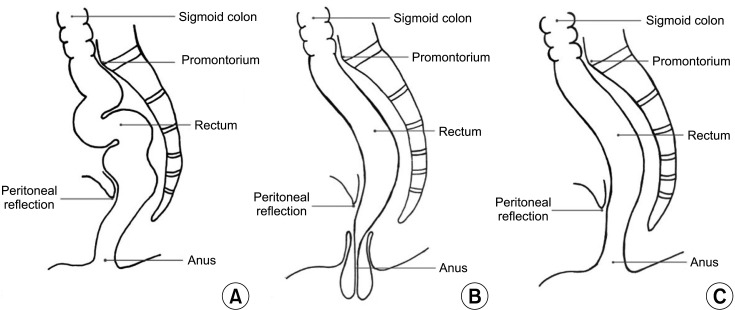
Rectal prolapse is a relatively rare disease that is estimated to affect less than 3 in 100,000 people [12]. It is associated with a range of risk factors and has several etiologies. It develops as a result of some factors including pregnancy, constipation, or chronic straining [3]. Rectal prolapse is more common in adults than children, and it is particularly prevalent in women aged 50 years or older, who are 6 times more likely to be affected than men [4]. Therefore, it is assumed that rectal prolapse develops as a consequence of multiple vaginal deliveries [13]. However, the precise cause of rectal prolapse is still unknown [356]. Few theories have been proposed regarding the pathophysiology of rectal prolapse [7]. For decades, it has been hypothesized that rectal prolapse develops due to laxity or weakness of the pelvic floor muscles [8]. Although the development of pelvic floor laxity in females is often supposed to be related to childbearing, 50% of females with rectal prolapse were reported to be nulliparous [6]. Furthermore, this hypothesis does not explain the rectal prolapse in males. Recently, we suggested a novel hypothesis to explain the pathophysiology of rectal prolapse [9]. Our suggestion is that the etiology of rectal prolapse is related to rectal redundancy (elongated and well-mobilized rectum) rather than laxity or weakness of the pelvic floor muscles (Fig. 1). This study aimed to evaluate the role of rectal redundancy in the pathophysiology of rectal prolapse.
Go to : 
This study protocol was approved by the Ethics Committee of Marmara University School of Medicine (No. 09.2021.94), and all patients provided written informed consent.
In this prospective study, consecutive 14 adult patients with rectal prolapse who underwent surgery in Marmara University Pendik Teaching and Research Hospital between December 2019 and February 2021 were included in the study group. To constitute a control group without selection bias, we included consecutive 17 patients who underwent laparotomy for reasons other than rectal prolapse in the control group. Details regarding the control group have been provided in the result section. Any sign of rectal prolapse has been defined as the sole exclusion criteria for the control group.
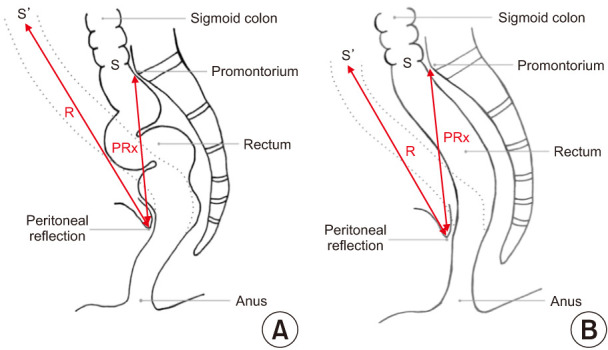
Regarding demographics, symptoms, previous history of childbearing, and history of abdominal or perineal surgery were collected prospectively. Rectal prolapse was evaluated preoperatively by physical examination and magnetic resonance imaging defecography. The Oxford rectal prolapse grading system was used for prolapse classification in this study [10]. The length of the intraperitoneal rectum, the distance between the promontorium and peritoneal reflection (PRx), the length of the mesorectum, and the length of the sigmoid colon were measured intraoperatively in both the study and control groups. The length of the intraperitoneal rectum was defined as the length of the rectal part between the promontorium and peritoneal reflection (Fig. 2A, B). Moreover, the length of the mesorectum was defined as the distance between the midrectum and the sacrum where the midrectum is attached (Fig. 3A, B). The length of the intraperitoneal rectum and the PRx were measured using a steel surgical ruler. The length of the mesorectum and the length of the sigmoid colon were measured using a soft silicone ruler. To measure the redundancy of the rectum, we calculated the ratio of the intraperitoneal rectum length to the PRx (R/PRx). R/PRx was considered an indicator for redundancy according to physics rules.
Data analysis was performed using IBM SPSS Statistics ver. 23.0 (IBM Corp., Armonk, NY, USA). The t-test or Mann-Whitney U-test was used to analyze continuous data. Fisher exact test or the chi-square test was used to analyze categorical data. All tests were 2-sided. The P-values less than 0.05 were considered statistically significant.
The primary outcome of this study was to evaluate whether R/PRx is higher in patients with rectal prolapse compared to the control group.
Go to : 
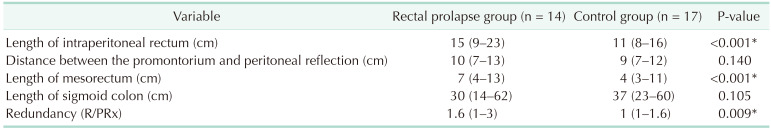
A total of 14 consecutive adult patients with rectal prolapse who underwent rectopexy in Marmara University Pendik Teaching and Research Hospital between December 2019 and February 2020 were included in the study group. Six patients (42.9%) were diagnosed as grade 5 (complete prolapse), 5 (35.7%) as grade 4, and 3 (21.4%) as grade 3. Mesh rectopexy with open surgery was performed for all the patients using the Wells procedure [11]. The control group consisted of 17 patients who underwent elective laparotomy for a reason other than rectal prolapse and had no symptoms of rectal prolapse or obstructive defecation. Ten patients with gastric cancer, 4 patients with right colon cancer, 2 patients with pancreas cancer, and 1 patient with ventral hernia, who all underwent laparotomy, were included in the control group. The median age, sex, body mass index, previous history of childbearing, and history of abdominal or perineal surgery were not different between the 2 groups. However, symptoms regarding rectal prolapse were significantly higher in the prolapse group (Table 1). Comparing the anatomic features revealed that the length of the sigmoid colon and PRx were not significantly different between the 2 groups. However, the length of the intraperitoneal rectum was significantly higher in the prolapse group. Furthermore, the median R/PRx in the prolapse group was significantly higher than in the control group (1.6 [range, 1.0–3.0] vs. 1.0 [range, 1.0–1.6], P = 0.009) (Table 2). This means that the intraperitoneal rectum in patients with rectal prolapse is significantly more redundant than in the normal population (Fig. 4A, B).
Go to : 
This study showed that the length of the intraperitoneal rectum was significantly longer and more redundant in patients with rectal prolapse than in the normal population.
In the prolapse group in this study, 35.7% of the patients were male and 22.2% were nulliparous females. Therefore, we think that parity and pelvic floor weakness might be less important than redundancy of the rectum. Redundancy is common and well-defined in the human colon. It was defined as an abnormally tortuous and long colon with additional loops or twists, particularly in the left colon. We think that this definition can be applied to the rectum. Therefore, this variation of the rectum may lead to obstructive defecation, which can be easily diagnosed in rectal prolapse. However, this diagnosis is usually underestimated in cases of internal intussusception. When defining redundancy in the rectum, we calculated the ratio of the length of the rectum to the promontory reflection distance rather than the absolute length of the rectum alone, and we evaluated it together with the length of the mesorectum. Furthermore, a long rectum may not be redundant, but may be straight and well-fixed to the sacrum. The pathophysiology of rectal prolapse is not well-defined. Few theories have been suggested to explain the cause of rectal prolapse. These theories include constipation, laxity of the rectal ligaments, deep pouch of the Douglas, rectal wall invagination, and laxity or weakness of the pelvic floor muscles [12].
Due to the fact that most patients with rectal prolapse have a long history of constipation, it is thought that extreme chronic straining during defecation may predispose to rectal prolapse [68]. However, we believe that rectal intussusceptions or prolapse lead to obstructive defecation, which may subsequently result in severe straining during defecation. Although the theory of laxity or weakness of the pelvic floor still seems valid, we do not think that pelvic floor weakness is the main cause of rectal prolapse. For example, abdominal wall weakness around a colostomy leads to parastomal hernia but not to stoma prolapse. Furthermore, an elongated non-fixed bowel may cause stoma prolapse without any weakness of the abdominal wall. In the same way, we think that an anatomic variation of a redundant (elongated) rectum may predispose to rectal prolapse without pelvic floor weakness. It has been reported that a redundant sigmoid colon is the most common anatomic feature associated with rectal prolapse [6]. However, this observational feature has not been proven by randomized controlled clinical trials. However, we demonstrated in this study that the length of the sigmoid colon was not significantly different in patients with rectal prolapse compare with the control group. According to our hypothesis, we think that a deep Douglas pouch and rectal wall intussusception are not causes of rectal prolapse, but the result of rectal redundancy, which leads to intussusception and subsequently to complete rectal prolapse. It has also been reported that loss of the vertical position of the rectum and its sacral attachments was another most common anatomic feature associated with rectal prolapse [6], and this has been proven in this study by objective measurements, since the loss of the vertical position of the rectum and its sacral attachments was measured by redundancy in this study. To date, over 100 surgical modalities were identified for rectal prolapse treatment. Transabdominal or perineal procedures are performed to repair rectal prolapse. Transabdominal repairs involve rectal fixation, rectal resection, or a combination of resection and fixation. Perineal procedures including the Altemeier operation (perineal proctosigmoidectomy) and the Delorme procedure in which transanal resection of the rectum or the rectal plication method is also used to shorten the length of the rectum [21213]. Both transabdominal and perineal procedures are performed to correct the redundancy of the rectum without any intervention to the pelvic floor or muscles, giving us a hint that rectal prolapse may not be the result of a pelvic floor or muscle pathology. To evaluate this hypothesis in an in vivo model, we previously conducted an animal study to evaluate the role of rectal redundancy in the pathophysiology of rectal prolapse, and we observed the occurrence of rectal prolapse in response to rectal mobilization in rats [13]. We think that rectal redundancy is an anatomic variation that predisposes to rectal prolapse. Therefore, further studies are needed to evaluate the prevalence of rectal redundancy in the population. In addition, further prospective randomized studies are needed to evaluate the role of rectal redundancy in the pathophysiology of rectal prolapse.
To the best of our knowledge, this is the first study that highlights this issue with a logical and measurable explanation of the etiology of rectal prolapse. Furthermore, the prospective design and presence of a control group are considered the strengths of this study. The small number of patients and lack of parameters regarding pelvic floor weakness are the limitations of this study.
In conclusion, this study showed that intraperitoneal rectum in patients with rectal prolapse is significantly more redundant than in the normal population. This could be considered reasonable evidence for the role of rectal redundancy in the pathophysiology of rectal prolapse.
Go to : 
References
1. Mills S. Chapter 33: Rectal prolapse. Beck DE, Roberts PL, Saclarides TJ, Senagore AJ, Stamos MJ, Wexner SD, editors. ASCRS textbook of colon and rectal surgery. 2nd ed. New York: Springer;2011.
2. Varma M, Rafferty J, Buie WD. Standards Practice Task Force of American Society of Colon and Rectal Surgeons. Practice parameters for the management of rectal prolapse. Dis Colon Rectum. 2011; 54:1339–1346. PMID: 21979176.
3. Goldstein SD, Maxwell PJ 4th. Rectal prolapse. Clin Colon Rectal Surg. 2011; 24:39–45. PMID: 22379404.
4. Sun C, Hull T, Ozuner G. Risk factors and clinical characteristics of rectal prolapse in young patients. J Visc Surg. 2014; 151:425–429. PMID: 25242503.
5. O’Brien DP 4th. Rectal prolapse. Clin Colon Rectal Surg. 2007; 20:125–132. PMID: 20011387.
6. Bordeianou L, Hicks CW, Kaiser AM, Alavi K, Sudan R, Wise PE. Rectal prolapse: an overview of clinical features, diagnosis, and patient-specific management strategies. J Gastrointest Surg. 2014; 18:1059–1069. PMID: 24352613.
7. Pucciani F. Rectal prolapse: pathophysiology. Altomare DF, Pucciani F, editors. Rectal prolapse. Milano: Springer, Milano;2008. p. 13–19.
8. Patcharatrakul T, Rao SS. Update on the pathophysiology and management of anorectal disorders. Gut Liver. 2018; 12:375–384. PMID: 29050194.
9. Attaallah W. Update on the pathophysiology of rectal prolapse. Turk J Gastroenterol. 2019; 30:1074–1075. PMID: 31258139.
10. Wijffels NA, Collinson R, Cunningham C, Lindsey I. What is the natural history of internal rectal prolapse? Colorectal Dis. 2010; 12:822–830. PMID: 19508530.
11. WELLS C. New operation for rectal prolapse. Proc R Soc Med. 1959; 52:602–603. PMID: 13843894.
12. Madoff RD, Mellgren A. One hundred years of rectal prolapse surgery. Dis Colon Rectum. 1999; 42:441–450. PMID: 10215042.
13. Attaallah W, Menek G, Erdaj FA, Inceoglu IY, Kankilic MP, Yilmazer AH, et al. An experimental study on the pathophysiology of rectal prolapse. Turk J Surg. 2021; 37:151–156.
Go to : 




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download