This article has been
cited by other articles in ScienceCentral.
Abstract
Purpose
After the publication of the ACOSOG (American College of Surgeons Oncology Group) Z0011 trial, the rate of axillary lymph node dissection has reduced. Thus, the need for intraoperative frozen section biopsy of sentinel lymph nodes (SLNs) has become controversial. We identified patients for whom intraoperative SLN frozen section biopsy could be omitted and found that frozen section biopsy rate can be reduced.
Methods
We reviewed the records of patients with tumors ≤5 cm in diameter who underwent breast-conserving surgery between January 2013 and December 2019 at Seoul St. Mary’s Hospital. Clinicopathological and imaging characteristics were compared according to number of positive SLNs (0–2 SLNs positive vs. ≥3 SLNs positive).
Results
A total of 1,983 patients were included in this study. Thirty-two patients (1.6%) had at least 3 positive SLNs. Patients with ≥3 positive SLNs had significantly larger tumors and were more frequently high-grade tumors (P < 0.001 and P = 0.002, respectively). Identification of suspicious lymph nodes on imaging studies was also associated with the presence of ≥3 positive SLNs (hazard ratio, 11.54; 95% confidence interval, 4.42–30.10). All patients with none or only 1 suspicious lymph node on any imaging modality (n = 647, 32.6%) had 0–2 positive SLNs. Also, among patients with clinical T1-stage tumors and at least 2 suspicious lymph nodes on only 1 imaging modality (n = 514, 25.9%), only 2 cases had ≥3 positive SLNs.
Conclusion
We found that intraoperative SLN frozen biopsy could be omitted in patients using tumor size and axillary lymph node status on imaging modality.
Keywords: Breast neoplasms, Frozen sections biopsy, Magnetic resonance imaging, Sentinel lymph node
INTRODUCTION
Axillary lymph node (ALN) metastasis is one of the most important prognostic factors for early breast cancer patients. Axillary management, which has evolved from ALN dissection (ALND) to sentinel lymph node (SLN) biopsy is essential for axillary staging and reducing surgical morbidity without compromising oncological safety. Frozen section biopsy is widely used to identify ALN metastases intraoperatively, reducing the need for a second operation when metastases are identified in SLNs.
Currently, the need for ALND has decreased because several clinical trials have established the safety of ALND omission in early breast cancer patients with minimal axillary involvement [
123]. Breast cancer patients with clinical T1–2 tumors, node-negative disease and those undergoing primary surgery are candidates for ALND omission. If patients’ evidence micro-metastases or only 1 or 2 metastatic SLNs, ALND can be omitted without compromising the oncological outcomes. Since the publication of these studies, the ALND rate has markedly decreased worldwide [
45]. This raises the question of whether intraoperative SLN frozen section biopsy is required. Frozen section biopsy exhibits low sensitivity in terms of micrometastasis detection and is associated with a risk of permanent tissue loss; it prolongs the operation time and increases medical costs. Hence, we aimed to identify preoperative status which affects SLNs positivity and patients for whom SLN frozen section biopsy can be omitted without increasing the risk of second operation for ALND.
METHODS
The study was approved by the Institutional Review Board of Seoul St. Mary’s Hospital (No. KC21RISI0774); the need to obtain informed patient consent was waived.
Patients who underwent breast cancer surgery from January 2013 to December 2019 at Seoul St. Mary’s Hospital were considered eligible. Patients with clinical stage T1–2 tumors who underwent breast-conserving surgery were reviewed retrospectively. Using the criteria of the ACOSOG (American College of Surgeons Oncology Group) Z0011 trial, patients who underwent total mastectomy or neoadjuvant chemotherapy, or who exhibited tumors of clinical stage T3–4, were excluded. Patients who underwent surgery to treat ductal carcinoma in situ, phyllodes tumor, occult breast cancer, or local recurrence and those who initially underwent ALND, who did not undergo any axillary surgery, or who were diagnosed preoperatively with ALN metastasis or internal mammary lymph node metastasis, were also excluded. Patients who underwent initial surgery in other hospitals were excluded as clinical tumor size data was missing.
Every case was evaluated in terms of clinicopathological factors as well as by breast ultrasonography, mammography, breast MRI, and PET/CT or chest CT. In mid-2017, the health insurance policy of the Republic of Korea stopped covering PET/CT and was replaced with chest CT; therefore, we combined PET/CT and CT data in the present study. We did not include physical examination data because of incomplete medical records.
Axillary imaging results were extracted from the radiology reports of board-certified breast radiologists. We used the breast imaging-reporting and data system (BI-RADS) to evaluate ultrasonographic, mammographic, and MRI data. An ALN was considered suspicious if it exhibited eccentric cortical thickening, a round shape, or absence of fatty hilum [
6]. An ALN with
18F-fluorodeoxyglucose uptake observed by PET/CT was defined as a suspicious ALN [
7]. The axillary imaging results were divided into 3 categories according to the number of suspicious lymph nodes: no suspicious ALN, 1 suspicious ALN, and at least 2 suspicious ALNs.
The clinical tumor size, tumor location, and multifocality status were determined by breast MRI, but we used ultrasound data when MRI results were lacking. Pathological parameters (histological subtype, tumor grade, hormone receptor status, and human epidermal growth factor receptor [HER2] and Ki67 status) were obtained from pathological analyses of surgical specimens. Commencing in mid-2017, ALND omission as recommended by the ACOSOG Z0011 and IBCSG (International Breast Cancer Study Group) 23-01 trials was partially performed.
We divided patients into 2 groups; 0–2 positive SLNs group and ≥3 positive SLNs group, to compare patients that could omit ALND to patients who should perform ALND. Clinicopathologic factors were also compared according to number of metastatic ALNs to explore whether factors related to positive SLNs sustained in these groups too.
Univariate analysis was performed using the Pearson chi-square test, Student t-test, and logistic regression analysis, as appropriate. Statistical significance was defined as a P-value of <0.05. All statistical analyses were conducted using IBM SPSS Statistics ver. 23.0 (IBM Corp., Armonk, NY, USA). Variables are presented as numbers with percentages.
RESULTS
A total of 4,301 patients underwent breast cancer surgery at Seoul St. Mary’s Hospital from January 2013 to December 2019 (
Fig. 1). After applying the exclusion criteria, 1,983 cases were included, among which 11 were bilateral breast cancer cases. The median patient age was 52 years (range, 14–88 years). More than 60% of cases had a clinical T1 tumor and the majority (n = 1,680, 84.7%) were invasive ductal carcinoma. ALND omission was performed in 187 cases (9.4%). The study cohort was divided into 2 groups according to SLN metastasis status: patients with less than 3 positive SLNs (0–2 positive SLNs) and those with at least 3 positive SLNs (≥3 positive SLNs). Thirty-two patients (1.6%) had ≥3 positive SLNs. The 2 groups were compared in terms of clinicopathological factors and radiological findings (
Table 1). Patients with ≥3 positive SLNs exhibited significantly larger tumors on preoperative MRI (P < 0.001), but age, multifocality, and tumor location did not affect the number of positive SLNs. High-grade tumors were more frequent in patients with ≥3 positive SLNs (P = 0.008), but histological subtype, hormone receptor status, HER2 status, and Ki67 were not associated with nodal status. The results were generally similar when patients were analyzed according to ALN metastasis status, with the exception that tumor location affected the ALN metastasis status (
Supplementary Table 1). Patients with at least 3 positive ALNs had a higher rate of upper, outer quadrant tumors compared with those with less than 3 positive ALNs (P = 0.011).
The radiological findings were categorized according to the number of suspicious ALNs revealed by the various imaging modalities. The number of suspicious ALNs was significantly related to the number of positive SLNs regardless of the imaging modality used (all P < 0.001). Notably, when MRI revealed no suspicious ALNs, no case had more than 2 positive SLNs. The radiological findings were also categorized according to the number of imaging modalities revealing several suspicious ALNs (
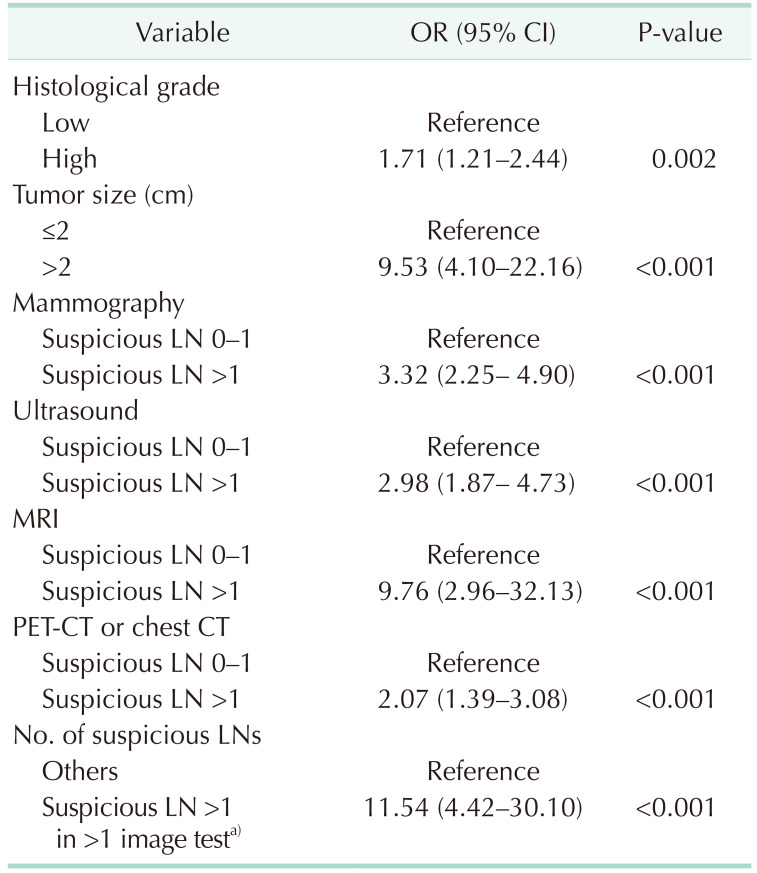
Table 2). All cases that were negative for ALNs on imaging studies (n = 528, 27.1%) or had only 1 suspicious ALN on all imaging modalities (n = 119, 6.1%) had 0–2 positive SLNs. On univariate logistic regression analysis, larger tumor size, 2 or more suspicious ALNs on a single-image test result, and 2 or more suspicious ALNs on multi-imaging modalities were associated with at least 3 positive SLNs group (
Table 3).
Patients who exhibited none or only 1 suspicious ALN on any imaging modality (n = 647, 32.6%) did not require intraoperative SLN frozen section biopsy; they were not at risk of a second operation featuring ALND. Also, in patients with clinical T1-stage tumors and at least 2 suspicious lymph nodes on only 1 imaging modality (n = 514, 25.9%), only 2 cases (0.4%) had more than 2 positive SLNs. Thus, if intraoperative SLN frozen section biopsy is omitted in these cases, 1,161 patients (58.5%) would not undergo unnecessary frozen section biopsy, and only 2 patients (0.1%) would require a second operation for ALND (
Fig. 2).
DISCUSSION
The ALND rate has decreased after several clinical trials demonstrated the oncological safety of ALND omission in patients with limited axillary nodal burden. Thus, the need for intraoperative SLN evaluation has also decreased. Here, we sought clinicopathologic factors identifying patients who do not need SLN frozen section biopsy without any increase in the risk of a secondary operation featuring ALND. Both tumor size and axillary imaging status were strongly associated with axillary nodal status. The MRI results and the number of suspicious lymph nodes evident on imaging studies could be considered when the possibility of omitting SLN frozen section biopsy is raised.
The need for intraoperative SLN frozen section biopsy has been discussed in several studies [
89101112]. Those studies focused principally on accuracy of frozen section biopsy, reporting a false-negative rate of 11.7%–13.6% [
91112]. Although micro-metastases greatly affect the false-negative rate, the IBCSG 23-01 trial found that micro-metastases are not an indication for additional ALND. The current role of intraoperative SLN frozen section biopsy remains controversial. In studies that actively omitted ALND (guided by the ACOSOG Z0011 trial data), the need for SLN frozen section biopsy was minimal [
1113]. However, studies that questioned the appropriateness of ALND omission argued the need for frozen section biopsy [
89111415].
In this study, we sought to identify patients who could safely omit SLN frozen section biopsy. Various nomograms and predictive models guiding selective frozen section biopsy of SLNs have been proposed [
1617]. However, the included factors differed from this study. The nomogram of Ahn et al. [
16] included axillary ultrasound and chest CT data and patient age. Our study also included preoperative imaging data, but additionally added MRI data that greatly reflected ALN burden in the study. Zeng et al. [
17] developed a negative binomial regression model that included histological grade, estrogen receptor, and HER2 status, but lacked imaging data which was an important factor in this study. Contrary to previous studies, age and estrogen receptor/HER2 status did not affect the ALN metastatic burden in this study.
Imaging is essential in terms of axillary evaluation and estimation of the nodal burden. Ultrasound is useful for evaluating axillae and is performed in newly diagnosed breast cancer at many centers. MRI reveals the breast cancer extent and yields additional axillary data, including enhancement of potentially suspicious lymph nodes. MRI can be used to compare the 2 axillae and is less operator-dependent than ultrasound [
18]. Moreover, MRI provides a global view of the axilla, which is increasingly relevant because the number of metastatic ALNs is more important than simply identifying metastasis. Notably, we found that the MRI results were strongly correlated with nodal burden. PET/CT is useful for evaluating the axillae and revealing metabolic activity. The diagnostic accuracies of PET/CT and MRI for axillary evaluation are comparable, but MRI affords a higher sensitivity when functional values are included [
1920].
This study had several limitations. This was a single-center retrospective study, and therefore selection bias may have been in play. We did not include physical examination data because the medical records lacked consistency; high-quality records were required when selecting patients for the ACOSOG Z0011 trial. Also, the tool used to evaluate disease extent changed from CT to PET/CT during the study period due to change of reimbursement policy. Additionally, although the 2 groups were homogeneous, the patient numbers differed considerably, which may have reduced the reliability of our results.
In conclusion, both preoperative imaging data of tumor size and ALNs status are important when predicting whether there will be more than 2 positive SLNs. SLN frozen section biopsy omission can be considered in patients with small tumors and minimally suspicious ALNs on imaging studies including breast MRI.
Fig. 1
Flowchart of the patients. ALND, axillary lymph node dissection; ALN, axillary lymph node; SLN, sentinel lymph node.
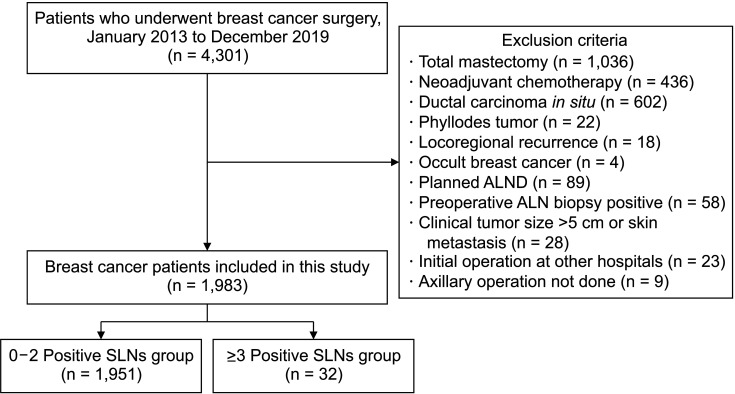
Fig. 2
Number of patients that can be considered of sentinel lymph node frozen section biopsy omission. Group 1, no or only 1 suspicious lymph node in all radiologic tests; group 2, clinical tumor size of ≤2 cm and 2 or more suspicious lymph nodes in only 1 radiologic test.
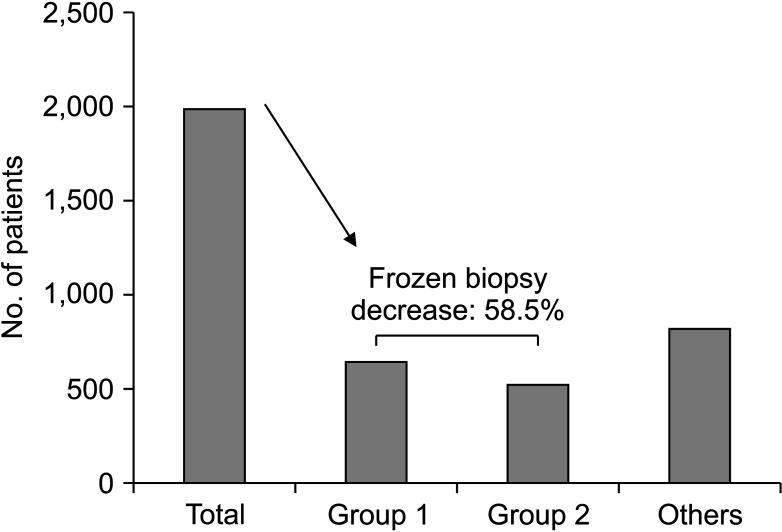
Table 1
Clinicopathological characteristics associated with number of positive SLNs
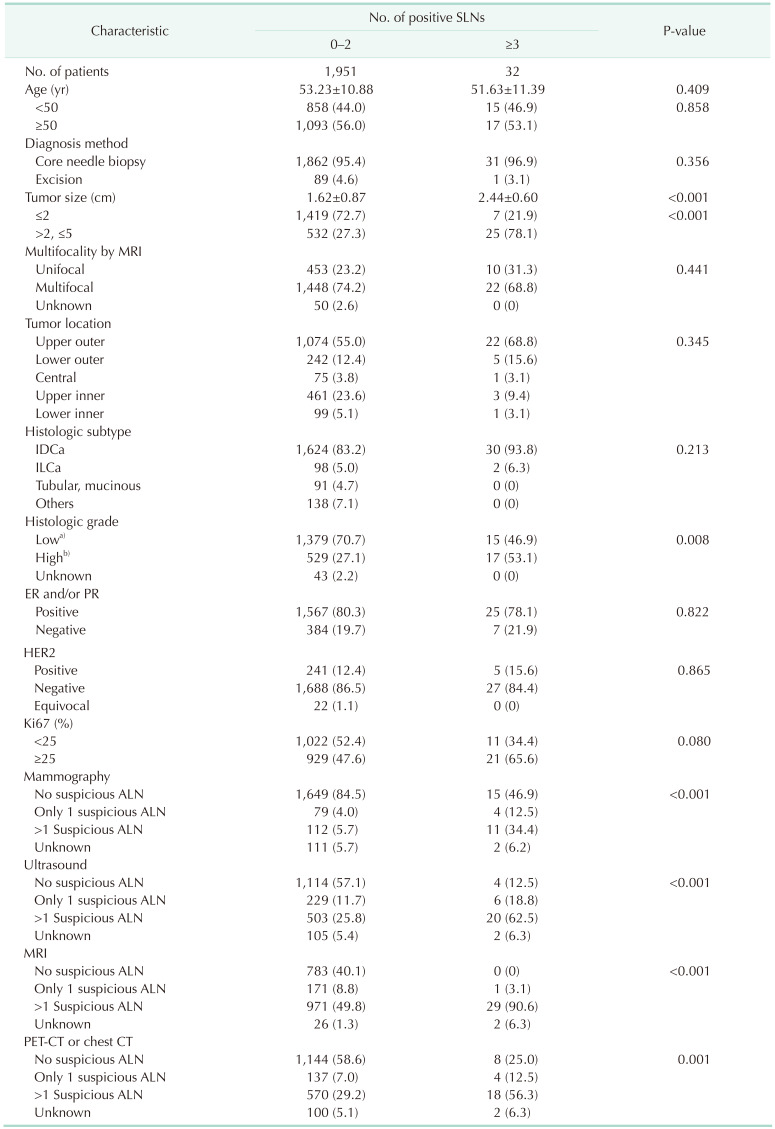
Table 2
Radiologic findings of suspicious LNs by number of positive SLNs
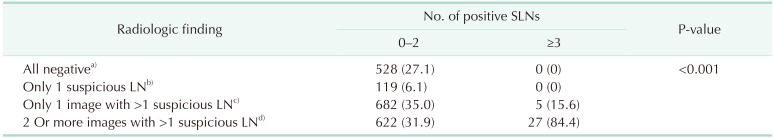
Table 3
Univariate analysis of predictive variables for 3 or more metastatic SLNs








 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download