Abstract
Purpose
For moderately advanced rectal cancers with safe circumferential margins, the oncologic benefit of neoadjuvant radiotherapy must be reconsidered because of the possibility of overtreatment, resulting in complications from radiotoxicity. To evaluate the oncologic safety of the omission of neoadjuvant radiotherapy for moderately advanced rectal cancers, we evaluated and compared the prognoses of patients who underwent radical resection with and without neoadjuvant radiotherapy for T2/N1 and T3N0/1 middle and low rectal cancers with safe circumferential resection margins.
Methods
We retrospectively enrolled 66 patients who underwent radical resection for clinical (c) T2N1 and T3N0/1 middle and low rectal cancers between 2008 and 2014. Patients with distant metastasis; cT4, cN2, or positive lateral pelvic lymph nodes; positive circumferential resection margin; signet-ring cell carcinoma; cT1/2N0; or those who had received adjuvant radiotherapy were excluded. The clinical and pathological characteristics and 5-year oncologic outcomes of the no-radiotherapy (n = 34) and radiotherapy (n = 32) groups were compared.
Results
The rates of abdominoperineal resection and ileostomies and the proportion of patients who received adjuvant chemotherapy were significantly higher in the radiotherapy group. There were no significant differences in tumor location, clinical stage, surgery type, pathologic N stage, anastomotic leakage, or long-term oncologic outcomes including 5-year disease-free survival, overall survival, and local recurrence and distant metastasis rates between both groups.
Go to : 
Neoadjuvant chemoradiotherapy followed by surgery is the current standard therapeutic strategy for locally advanced (defined as clinical stage of T3 or above, or node-positive) middle or low rectal cancer based on its superiority for local control [123].
However, due to radiotoxicity, neoadjuvant radiotherapy (RT) can increase the rate of anastomotic failure and the necessity of stoma and sometimes stoma may be maintained permanently. Keeping a stoma and its closure may also result in complications. RT can affect defecation [4], urinary or sexual function [5] especially in the proctectomy state, which can disturb the quality of life of patients significantly and sometimes irreversibly. In addition, because long-course RT requires a long therapeutic duration of more than 14 weeks including the resting period, radical resection and adjuvant treatment are delayed and patients can feel anxious about this.
For rectal cancers threatening or including the circumferential margin or invading adjacent organs, neoadjuvant RT is necessary for successful R0 resection and better local control. For locally advanced rectal cancers with extensive lymph node metastases or extramural venous invasion which require not only local control but also systemic control, total neoadjuvant treatment (TNT) can be considered [6].
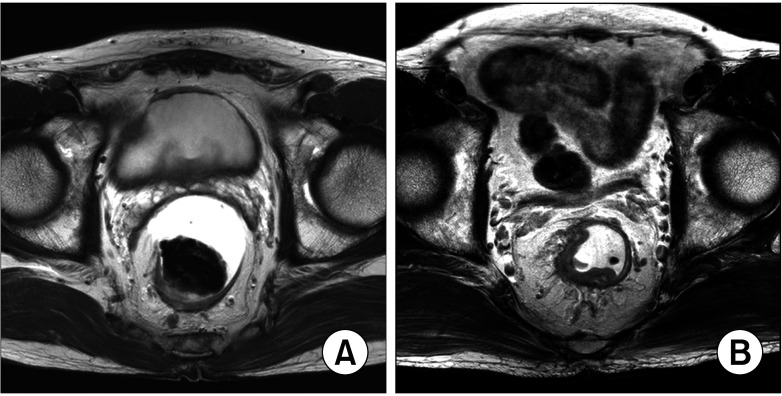
However, for moderately advanced cancers, such as clinical T2N1 or T3N0/1, which has safe circumferential resection margins (Fig. 1), the oncologic benefit of neoadjuvant RT needs to be reconsidered because it may not outweigh its surgical, functional, and oncological harmful effects. Considering the improvement and standardization of total mesorectal excision (TME) via technical development and worldwide education, some rectal cancers that required RT in the past may need only resection for local control. Additionally, clinically overstaged cancers that are not indicated based on pathologic staging can be overtreated [7]. Therefore, the conventional indication for neoadjuvant chemoradiotherapy (NCRT) needs to be reestablished to exclude patients who do not require neoadjuvant RT and prevent unnecessary complications.
To evaluate the oncologic safety of the omission of NCRT for moderately advanced rectal cancers, we evaluated and compared the prognoses of patients who underwent radical resection with and without NCRT for T2/N1 and T3N0/1 middle and low rectal cancers with safe circumferential resection margins.
Go to : 
The approval for this retrospective study protocol was obtained from the Institutional Review Board of Chungnam National University Hospital (No. 2021-10-034). The requirement for informed consent was waived because of the retrospective nature of the study.
The electronic medical records of patients who underwent radical surgery for rectal cancer between 2008 and 2014 were retrospectively evaluated. The inclusion criteria were as follows: middle or lower rectal cancer; diagnosis of clinical T2N1 or T3N0/1 based on the initial imaging study including abdominopelvic CT, rectal MRI, or PET; radical low anterior resection, intersphincteric resection, abdominoperineal resection; and successful R0 resection. The exclusion criteria were tumors threatening or including the circumferential resection margin or invading adjacent organs; clinical N2 stage or positive lateral pelvic lymph node; distant metastasis; clinical T1/2N0 stage; histologically signet-ring cell carcinoma; and adjuvant RT. Thirty-four and 32 patients were enrolled in the no-RT group and the RT group, respectively.
The initial diagnosis and clinical staging were based on the findings of colonoscopy with biopsy, abdominopelvic CT, chest CT, transrectal ultrasonography, and rectal MRI. Clinical staging was performed by gastrointestinal radiologists based on a 2016 consensus recommendation from the Korean Society of Abdominal Radiology [8]. If there was a discontinuity in the muscularis propria with extension of the tumor in the mesorectum, or suspicious of lymph node metastasis according to size (>8 mm of short-axis diameter or >10 mm of maximal diameter) and morphological features (including margin irregularity, heterogeneity of nodal texture, and shape), the patient was initially informed of NCRT as the standard guideline for locally advanced rectal cancer.
If the patient agreed, conventional chemoradiotherapy was performed. RT was delivered to the whole pelvic region by using a 3-field approach at a daily dose of 1.8 grays (Gy) in 25 fractions, followed by a boost of 5.4 Gy in 3 fractions, 5 days a week, for 6 weeks, with a total dose of 54 Gy. Oral capecitabine as a radiosensitizer was administered at a dose of 825 mg/m2 twice daily for 6 weeks along with RT. After at least 8 weeks from the end of NCRT, radical resection with total or tumor-specific mesorectal excision was performed.
However, when the patients refused neoadjuvant therapy for any reason as underlying medical condition (total of 9 including chronic kidney disease [n = 2], dementia [n = 2], cerebral infarction [n = 1], disability due to previous traffic accident [n = 1], previous pelvic irradiation for cervical cancer [n = 1], continuous rectal cancer bleeding [n = 1], and combined advanced gastric cancer [n = 1]) and personal situation (total of 25 including poor access or difficult daily-visit to the hospital, or preference for immediate surgery), upfront radical resection was performed if the surgeon also considered it feasible. A temporary ileostomy was created based on considerations of the individual risk factors for anastomotic leakage in both the no-RT group and the RT group. The administration of adjuvant chemotherapy with FOLFOX (fluorouracil, folinic acid, and oxaliplatin) or oral capecitabine was based on the pathologic stage and the conditions of the patients in both groups.
After the end of adjuvant treatment, regular surveillance with CEA, abdominopelvic CT, and plain chest radiography or chest CT were performed every 4 months for 2 years and every 6 months after 2 years. If recurrence was suspected during regular surveillance, further personalized evaluation, such as PET or MRI for the corresponding site, or surgical or percutaneous biopsy, was performed for confirmation. Personalized treatment for recurrence, such as palliative chemotherapy with or without radical resection, was administered.
The clinical, surgical, and pathological characteristics of the 2 groups were compared using the chi-square test. The 5-year oncologic outcomes, including disease-free survival (DFS), overall survival (OS), distant metastasis rate, and local recurrence rate of the 2 groups were analyzed and compared using Kaplan-Meier survival analysis and the log-rank test (P < 0.05). The analyses were performed using IBM SPSS Statistics ver. 20 (IBM Corp., Armonk, NY, USA).
Go to : 
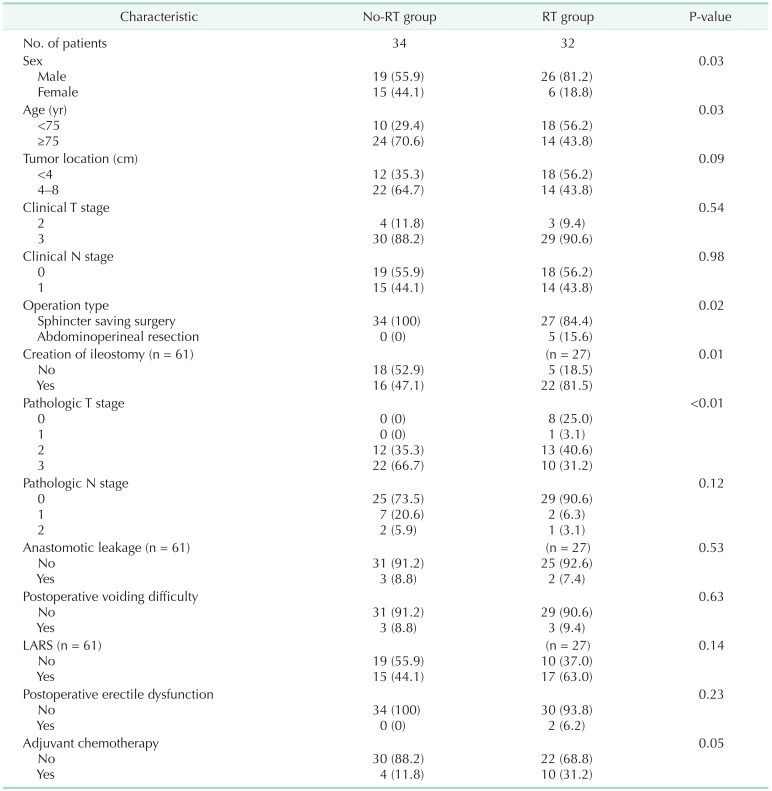
The clinical and pathologic characteristics are presented in Table 1. The RT group included more males and patients younger than 75 years old and had higher rates of abdominoperineal resection and ileostomies (P = 0.03, P = 0.03, P = 0.02, and P = 0.006, respectively). The prevalence of lower rectal cancer below 4 cm from the anal verge was higher in the RT group, but the difference was not significant. There were no significant differences in the rates of the clinical T and N stages between the 2 groups. While the rate of the pathologic T stage was significantly lower in the RT group than in the no-RT group (P < 0.001), there was no significant difference in the rate of the pathologic N stage between the 2 groups.
There were no significant differences in the rates of anastomotic leakage, postoperative voiding difficulty, low anterior syndrome, and postoperative erectile dysfunction between the 2 groups. The proportion of patients who received adjuvant chemotherapy was higher in the RT group with borderline significance (P = 0.05).
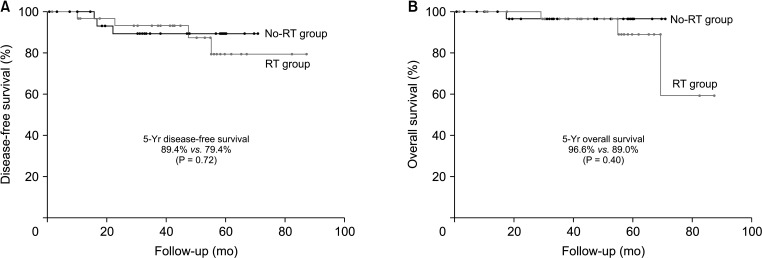
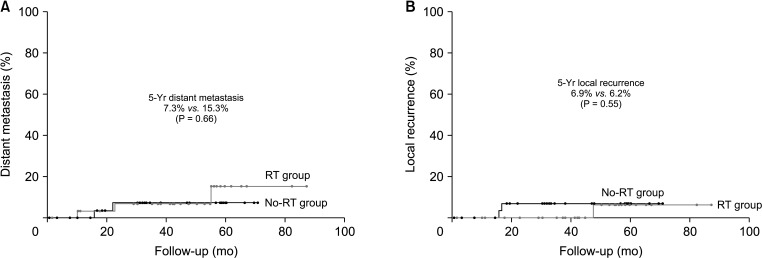
There were no significant differences in the 5-year oncologic outcomes, including DFS (89.5% in the no-RT group vs. 79.4% in the RT group, P = 0.72), OS (96.6% in the no-RT group vs. 89.0% in the RT group, P = 0.40), distant metastasis rate (7.3% in the no-RT group vs. 15.3% in the RT group, P = 0.66), and local recurrence rate (6.9% in the no-RT group vs. 6.2% in the RT group, P = 0.55) between the 2 groups (Figs. 2, 3). In the no-RT group, 2 patients had local recurrence. One had recurrence at the anastomosis 17 months after low anterior resection and underwent abdominoperineal resection. Another who showed signet-ring cell carcinoma and also had history of cervical cancer treated with pelvic irradiation before diagnosis and treatment of rectal cancer had local recurrence at the base of bladder and also peritoneal metastases 17 months after low anterior resection, and had only best supportive care. In the RT group, 1 patient had local recurrence. He had local recurrence at the presacral space 46 months after NCRT and abdominoperineal resection and underwent palliative chemotherapy. All of them expired due to disease progression.
In the present study, the proportion of male patients was significantly higher in the RT group than in the no-RT group. The concern for more technically challenging surgery in male patients may affect surgeons, and neoadjuvant RT may be preferable for males rather than upfront surgery for males. The proportion of patients younger than 75 years of age was also significantly higher in the RT group than in the no-RT group, and older patients with low performance scores tended to refuse neoadjuvant RT, which demands daily visits to the hospital for 6 weeks. Abdominoperineal resection was performed more frequently in the RT group, and this can be attributed to the difficulty in omitting RT for patients with rectal cancer close to or invading the anal canal. Although the prevalence of lower rectal cancer was higher in the RT group, the difference was not significant. An ileostomy was created more frequently in the RT group because RT is a risk factor for anastomotic leakage. However, the anastomotic leakage rates of both groups were similar, which may be attributed to the small sample of patients in the present study. The prevalence of the pathologic T stage was significantly lower in the RT group than in the no-RT group, and this was inevitable due to the local effect of RT. However, the pathologic N stages of both groups were similar, and 73.5% of the no-RT group had no lymph node metastases. This may suggest that the effect of neoadjuvant RT for local control of lymph node metastasis is not beneficial for cN1 rectal cancer. There were no significant differences in the rates of postoperative voiding difficulty and low anterior resection syndrome between the 2 groups. Postoperative erectile dysfunction only occurred in the RT group; however, the difference was not significant. The rate of adjuvant chemotherapy was higher, with borderline significance, for the RT group. However, there were no significant differences in oncologic outcomes, including 5-year DFS, OS, distant metastasis, and local recurrence between the 2 groups. Five-year DFS and OS were higher in the no-RT group, the distant metastasis rate was lower in the no-RT group, and the local recurrence rate was similar in both groups. This may suggest that neoadjuvant local treatment is unnecessary for moderately advanced rectal cancer, or it may have negative effects. The oncologic outcomes except for local recurrence of both groups were relatively favorable compared to previously reported literatures, which is considered because their stages were selected as clinical T2/N1 and T3N0/1.
Go to : 
In 2001, the Swedish rectal cancer trial [9] demonstrated that preoperative short-course RT combined with standardized TME, compared with surgery alone, improved local control. In 2004, the German CAO/ARO/AIO-94 trial [1] demonstrated that preoperative RT improved local control with less toxicity than postoperative RT. Based on this evidence, preoperative RT combined with TME followed by adjuvant chemotherapy has been the standard treatment for locally advanced rectal cancer, which is defined as a tumor beyond the rectal muscle or tumor with lymph node metastases.
Despite the oncologic benefits of RT for local control, it has numerous adverse effects. Irradiation induces tissue edema and fibrosis, disturbs surgical procedures and wound healing, and increases the rate of anastomotic leakage and anastomotic stricture [1011]. Some patients have to maintain a permanent stoma despite their favorable oncologic outcomes. In addition, irradiation deteriorates pelvic muscular and nervous structures and, consequently, impairs anorectal and genitourinary functions [12]. Irradiation aggravates the symptoms of low anterior resection syndrome following proctectomy [13]. Sometimes, delayed anastomotic leakage or a fistula between the rectum and anterior pelvic organs can occur several years after surgery due to irreversible radiotoxicity [14]. Bruheim et al. [15] reported that RT was associated with considerable longterm adverse effects on anorectal function and social function and a significant decrease in the quality of life.
Conventional RT requires 6 weeks of treatment and an 8-week resting period. Radical resection and systemic treatment should be delayed for periods of more than 14 weeks and can have negative effects on oncologic outcomes. Recently, the introduction of TNT with short-course RT and chemotherapy can be an option for patients who need preoperative local control, as well as prompt systemic treatment [16]. However, some patients with moderately advanced rectal cancer indicated for neoadjuvant treatment based on the conventional criteria may have no oncologic benefit, and it can be unnecessary and associated with harm from radiotoxicity. The history of neoadjuvant RT can be an indication for postoperative chemotherapy because it is considered the standard treatment for locally advanced rectal cancer regardless of its pathologic stage. In the present study, the rate of adjuvant chemotherapy was significantly higher in the RT group than in the no-RT group despite their similar stage, and oncologic results of the 2 groups were similar despite the higher rate of adjuvant chemotherapy in the RT group.
Not only overtreatment based on clinical over-staging but also down-staging by RT can affect the prognosis and the decision about optimal adjuvant treatment due to the obscure initial stage. If microscopically existing lymphovascular invasion or extramural venous invasion disappears after RT, the risk factors for metastasis may not be identified. Moderately advanced rectal cancers with controversy regarding the necessity of adjuvant chemotherapy need to be more precisely evaluated to predict prognosis and improve oncologic outcomes. Adjuvant chemotherapy is omitted or reduced more frequently and easily in good responders to neoadjuvant RT; however, it does not improve OS [12]. Even if some locally advanced rectal cancers showed a good response to local treatment, systemic control may be necessary. Therefore, down-staging by neoadjuvant RT without significant benefit can negatively affect survival outcomes because of inappropriate omission of adjuvant treatment.
After the standardization of preoperative RT plus TME, the Swedish rectal cancer trial reported a 9% long-term local recurrence rate in 2005 [17], the Dutch trial reported a 5-year local recurrence rate of 4.6% in 2010 [18], and the German CAO/ARO/AIO-94 trial reported an 11-year local recurrence rate of 7.1% in 2012 [19]. TME has already been standardized for rectal cancer surgery; however, there have been continuous advances due to the introduction of ultra-low anterior resection, intersphincteric resection, lateral pelvic lymph node dissection, the extralevator procedure [20], the development of laparoscopic and robotic techniques, and the transanal approach. Based on this, some moderately advanced rectal cancers that would have required RT to improve local control in the past may be treated with surgery only today. Specialized surgical techniques and accumulated surgical experiences have been reported to be associated with improved local control [21]. Within the last decade, several studies have reported that postoperative RT for stage II/III rectal cancer did not reduce recurrence or mortality [2223]. A prospective multicenter stratified randomized trial reported a significantly low incidence of local recurrence and favorable survivals were achieved by surgery alone in the low-risk group of stage II/III rectal cancers [24]. They suggested the discriminative use of neoadjuvant RT based on the clinical risk stratification for locally advanced rectal cancer. Jootun et al. [25] reported that neoadjuvant RT reduced local recurrence only in margin-threatening rectal cancer, and this strategy may reduce the toxicity of RT and allow an earlier introduction of systemic chemotherapy. Another population-based cohort study and a systematic review with meta-analyses have reported the importance of the circumferential resection margin as an independent prognostic factor based on the most recent literature [2627]. Therefore, the decision to use neoadjuvant RT for stage II/III rectal cancers needs to be based not only on the T and N stages but also on the circumferential resection margins.
The preoperative staging of rectal cancer has become more precise owing to the remarkable advances of MRI [28]. Pelvic MRI is the most accurate in determining the locoregional clinical staging of rectal cancers [29]. MRI enabled the sub-staging of T3 cancers into T3a to T3d based on the length of invasion of perirectal tissue and determination of the distance between the tumor and the circumferential resection margin. Most major trials that have proved the superiority of neoadjuvant RT performed CT and endorectal ultrasonography for initial staging [2919]. Therefore, the criteria for selecting patients who may benefit from neoadjuvant RT needs to be reestablished based on a more precise staging system with MRI in the present clinical setting. If the evidence is accumulated, it is considered mrT3aN0 could be excluded from the indication for NCRT.
On the contrary to this strategy of omission of NCRT in moderately advanced rectal cancer, NCRT can be considered rather for watch and wait approach with omission of radical surgery. Although the rates of clinical complete remission (cCR) are reported higher in moderate advanced rectal cancer compared to those of far advanced rectal cancer, the number of patients who do not acquire cCR is still more than the number of patients who acquire cCR, and tumor regrowth in patients who showed cCR after NCRT was reported as about 25% [30]. Therefore, even if watch and wait after NCRT can be an option for the early tumor to avoid proctectomy, radical surgery is considered as standard treatment for the tumor which is not early. In these patients with moderately advanced rectal cancer, omission of NCRT is considered to be safer oncologically compared to the omission of radical surgery.
Recently, TNT is recommended as the preferred strategy in locally advanced rectal cancer according to the National Comprehensive Cancer Network guidelines [3]. It has been reported to increase the rate of pathological complete remission and to be related to improved oncologic outcomes [6]. It needs to be considered preferentially for far advanced rectal cancers which require upfront systemic control. However, even if TNT could improve the oncologic outcomes in locally advanced rectal cancer, it is still necessary to select patients who acquire little oncological benefit from RT at cost of radiotoxicity.
In the era of personalized medicine, the treatment of locally advanced rectal cancer also has to be tailored. For the patients who do not fit for radical surgery because of their underlying condition or the patients who need local or systemic control before surgery, conventional or TNT need to be considered at first to achieve cCR or resectability. For the patient whose rectal cancer was moderately advanced as clinical T2/N1 and T3N0/1 with safe circumferential resection margins, even if they are indicated for neoadjuvant treatment according to the conventional criteria, upfront radical surgery and omission of RT can be considered.
The present study has limitations related to the small number of patients, retrospective analysis, and consequent heterogeneity of adjuvant treatment. Actually, the rate of lower rectal cancer and abdominoperineal resection were both higher in the RT group and this selection bias can be responsible for the no-RT group having higher rectal cancer with less necessity for RT. Therefore, further prospective randomized trials with larger samples are necessary to demonstrate the oncologic safety of omission of neoadjuvant RT.
In conclusion, the oncologic benefit of neoadjuvant RT for clinical T2/N1 and T3N0/1 middle and low rectal cancers with safe circumferential resection margins is considered to be unclear, and we suggest it can be omitted to prevent radiotoxicity and facilitate prompt essential treatment.
Go to : 
Notes
The abstract of this study has been presented as a virtual ePoster at the 2020 American Society of Colon and Rectal Surgeons (ASCRS) Annual Scientific Meeting held from June 6 to 10, 2020 in Boston, USA.
Go to : 
References
1. Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004; 351:1731–1740. PMID: 15496622.
2. Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006; 355:1114–1123. PMID: 16971718.
3. National Comprehensive Cancer Network (NCCN). NCCN Guidelines: rectal cancer, 2022 [Internet]. Plymouth Meeting, PA: NCCN;2022. cited 2022 Feb 17. Available from: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1461
.
4. Emmertsen KJ, Laurberg S. Low anterior resection syndrome score: development and validation of a symptom-based scoring system for bowel dysfunction after low anterior resection for rectal cancer. Ann Surg. 2012; 255:922–928. PMID: 22504191.
5. Bregendahl S, Emmertsen KJ, Lindegaard JC, Laurberg S. Urinary and sexual dysfunction in women after resection with and without preoperative radiotherapy for rectal cancer: a population-based cross-sectional study. Colorectal Dis. 2015; 17:26–37. PMID: 25156386.
6. Petrelli F, Trevisan F, Cabiddu M, Sgroi G, Bruschieri L, Rausa E, et al. Total neoadjuvant therapy in rectal cancer: a systematic review and meta-analysis of treatment outcomes. Ann Surg. 2020; 271:440–448. PMID: 31318794.
7. Horvat N, Carlos Tavares Rocha C, Clemente Oliveira B, Petkovska I, Gollub MJ. MRI of rectal cancer: tumor staging, imaging techniques, and management. Radiographics. 2019; 39:367–387. PMID: 30768361.
8. KSAR Study Group for Rectal Cancer. Essential items for structured reporting of rectal cancer MRI: 2016 consensus recommendation from the Korean Society of Abdominal Radiology. Korean J Radiol. 2017; 18:132–151. PMID: 28096724.
9. Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001; 345:638–646. PMID: 11547717.
10. Pommergaard HC, Gessler B, Burcharth J, Angenete E, Haglind E, Rosenberg J. Preoperative risk factors for anastomotic leakage after resection for colorectal cancer: a systematic review and meta-analysis. Colorectal Dis. 2014; 16:662–671. PMID: 24655784.
11. Qin Q, Ma T, Deng Y, Zheng J, Zhou Z, Wang H, et al. Impact of preoperative radiotherapy on anastomotic leakage and stenosis after rectal cancer resection: post hoc analysis of a randomized controlled trial. Dis Colon Rectum. 2016; 59:934–942. PMID: 27602924.
12. Mannaerts GH, Schijven MP, Hendrikx A, Martijn H, Rutten HJ, Wiggers T. Urologic and sexual morbidity following multimodality treatment for locally advanced primary and locally recurrent rectal cancer. Eur J Surg Oncol. 2001; 27:265–272. PMID: 11373103.
13. Jimenez-Gomez LM, Espin-Basany E, Trenti L, Martí-Gallostra M, Sánchez-García JL, Vallribera-Valls F, et al. Factors associated with low anterior resection syndrome after surgical treatment of rectal cancer. Colorectal Dis. 2018; 20:195–200.
14. Shin US, Kim CW, Yu CS, Kim JC. Delayed anastomotic leakage following sphincter-preserving surgery for rectal cancer. Int J Colorectal Dis. 2010; 25:843–849. PMID: 20387070.
15. Bruheim K, Guren MG, Skovlund E, Hjermstad MJ, Dahl O, Frykholm G, et al. Late side effects and quality of life after radiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2010; 76:1005–1011. PMID: 19540058.
16. Cercek A, Roxburgh CS, Strombom P, Smith JJ, Temple LK, Nash GM, et al. Adoption of total neoadjuvant therapy for locally advanced rectal cancer. JAMA Oncol. 2018; 4:e180071. PMID: 29566109.
17. Folkesson J, Birgisson H, Pahlman L, Cedermark B, Glimelius B, Gunnarsson U. Swedish Rectal Cancer Trial: long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol. 2005; 23:5644–5650. PMID: 16110023.
18. Kusters M, Marijnen CA, van de Velde CJ, Rutten HJ, Lahaye MJ, Kim JH, et al. Patterns of local recurrence in rectal cancer; a study of the Dutch TME trial. Eur J Surg Oncol. 2010; 36:470–476. PMID: 20096534.
19. Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012; 30:1926–1933. PMID: 22529255.
20. West NP, Finan PJ, Anderin C, Lindholm J, Holm T, Quirke P. Evidence of the oncologic superiority of cylindrical abdominoperineal excision for low rectal cancer. J Clin Oncol. 2008; 26:3517–3522. PMID: 18541901.
21. Martling AL, Holm T, Rutqvist LE, Moran BJ, Heald RJ, Cedemark B. Effect of a surgical training programme on outcome of rectal cancer in the County of Stockholm. Stockholm Colorectal Cancer Study Group, Basingstoke Bowel Cancer Research Project. Lancet. 2000; 356:93–96. PMID: 10963244.
22. Huh JW, Lim SW, Kim HR, Kim YJ. Effects of postoperative adjuvant radiotherapy on recurrence and survival in stage III rectal cancer. J Gastrointest Surg. 2011; 15:963–970. PMID: 21479672.
23. Park IJ, Kim HC, Yu CS, Kim TW, Jang SJ, Kim JC. Effect of adjuvant radiotherapy on local recurrence in stage II rectal cancer. Ann Surg Oncol. 2008; 15:519–525. PMID: 17960464.
24. Deng X, Liu P, Jiang D, Wei M, Wang X, Yang X, et al. Neoadjuvant radiotherapy versus surgery alone for stage II/III mid-low rectal cancer with or without high-risk factors: a prospective multicenter stratified randomized trial. Ann Surg. 2020; 272:1060–1069. PMID: 31599809.
25. Jootun N, Sengupta S, Cunningham C, Charlton P, Betts M, Weaver A, et al. Neoadjuvant radiotherapy in rectal cancer: less is more? Colorectal Dis. 2020; 22:261–268. PMID: 31556218.
26. Agger EA, Jörgren FH, Lydrup MA, Buchwald PL. Risk of local recurrence of rectal cancer and circumferential resection margin: population-based cohort study. Br J Surg. 2020; 107:580–585. PMID: 32133651.
27. Detering R, Rutgers MLW, Bemelman WA, Hompes R, Tanis PJ. Prognostic importance of circumferential resection margin in the era of evolving surgical and multidisciplinary treatment of rectal cancer: a systematic review and meta-analysis. Surgery. 2021; 170:412–431. PMID: 33838883.
28. Brown G, Davies S, Williams GT, Bourne MW, Newcombe RG, Radcliffe AG, et al. Effectiveness of preoperative staging in rectal cancer: digital rectal examination, endoluminal ultrasound or magnetic resonance imaging? Br J Cancer. 2004; 91:23–29. PMID: 15188013.
29. Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017; 28(Suppl 4):iv22–iv40. PMID: 28881920.
30. Marchegiani F, Palatucci V, Capelli G, Guerrieri M, Belluco C, Rega D, et al. Rectal sparing approach after neoadjuvant therapy in patients with rectal cancer: the preliminary results of the ReSARCh trial. Ann Surg Oncol. 2022; 29:1880–1889. PMID: 34855063.
Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download