Abstract
More than 2 years after the explosion of the coronavirus disease 2019 (COVID-19) pandemic, extensive efforts have been made to develop safe and efficacious vaccines against infections with severe acute respiratory syndrome coronavirus 2. The pandemic has opened a new era of vaccine development based on next-generation platforms, including messenger RNA (mRNA)-based technologies, and paved the way for the future of mRNA-based therapeutics to provide protection against a wide range of infectious diseases. Multiple vaccines have been developed at an unprecedented pace to protect against COVID-19 worldwide. However, important knowledge gaps remain to be addressed, especially in terms of how vaccines induce immunogenicity and efficacy in those who are elderly. Here, we discuss the various vaccine platforms that have been utilized to combat COVID-19 and emphasize how these platforms can be a powerful tool to react quickly to future pandemics.
According to the World Health Organization (WHO), as of December 2021, there have been more than 260 million confirmed coronavirus disease 2019 (COVID-19) cases, of which over 5 million have resulted in death, and over 7.9 billion doses of vaccines administered worldwide [1]. The Americas remain the region with the greatest number of confirmed COVID-19 cases, accounting for approximately 40% of all reported cases. In the Republic of Korea, over 4,077 of the 496,584 confirmed cases of COVID-19 have resulted in death, and over 42 million vaccine doses have been administered. Due to the rapid global spread of COVID-19, vaccines have been highlighted as the most effective countermeasure to protect the immunocompromised and induce herd immunity to maintain the rate of infection below the transmission threshold.
COVID-19 is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a positive-strand RNA coronavirus belonging to the Coronaviridae family. Its viral genome encodes structural and nonstructural proteins, including the following: nucleocapsid (N), spike (S), membrane (M), and envelope (E) proteins [2]. The SARS-CoV-2 S protein binds to angiotensin-converting enzyme 2 (ACE2), a receptor that is expressed in virtually all organs, including the lungs. Consequently, SARS-CoV-2 can infect more than the respiratory system to cause adverse effects and lead to highly variable host immune responses. The broad biodistribution of SARS-CoV-2 suggests that an ideal vaccine will need to elicit both immunoglobulin (Ig) A and IgG antibodies to protect the mucosal surface of the lungs and prevent systemic circulation of the virus [3].
Starting in early 2020, variants of SARS-CoV-2 emerged to pose an increased threat to global public health, further highlighting the priority of addressing the COVID-19 pandemic with safe and effective prophylactic and therapeutic strategies. Currently, the following five variants of SARS-CoV-2 have been classified as variants of concern (VOCs) by the WHO: alpha (B.1.1.7), beta (B.1.351), gamma (P.1), delta (B.1.617.2), and omicron (B.1.1.529) [4]. VOCs are assessed according to the degree of significance they have on global public health in terms of increased transmissibility, increased virulence, and whether the variants pose a threat to the currently available diagnostic procedures, vaccines, and therapeutic measures. The omicron (B.1.1.529) variant, which was designated as a VOC by the WHO in November 2021, possesses multiple mutations, including those in the spike protein, which have made the variant highly divergent, and COVID-19 vaccines that are currently available confer reduced protection against infection and symptomatic disease due to the omicron variant [5]. More importantly, the omicron variant has been declared the predominant strain among emerging COVID-19 cases in the United States [6]. Several lineages of the omicron variant have currently been identified and further monitoring is necessary to improve the preparedness and response strategies for addressing current and future variants of COVID-19.
COVID-19 vaccine development has relied on multiple platforms to combat the pandemic. Here, we discuss different strategies of COVID-19 vaccines, including traditional vaccine development strategies based on whole-virus vaccines, live or attenuated, in addition to technologies based on nucleic acid, viral protein subunit, and nonreplicating viral vectors (Fig. 1).
Virus-based vaccines include live-attenuated and inactivated viruses, which lack pathogenic characteristics and cannot mount a complete infection, respectively. Protein-based vaccines consist of purified proteins from viruses or infected cells, recombinant proteins, or virus-like particles, the latter of which are composed of structural proteins capable of forming virus particles without the viral genome.
Advances in molecular biology and vaccinology have also created novel platform technologies for vaccine development. These next-generation platforms rely on viral genome sequences that encode viral proteins, rather than the virus itself, for vaccine development, which is more adaptable for mass production during public health emergencies, such as the COVID-19 pandemic. Table 1 outlines the advantages and disadvantages of various vaccine platforms that have been utilized for the development of COVID-19 vaccines. Reports have indicated that the currently available vaccines are associated with minor side effects, including headache, nausea, and pain/soreness at the injection site, although rare and unusual adverse events have also been observed for specific types of COVID-19 vaccines [7].
Inactivated vaccines employ viruses that can no longer replicate because of heat or chemical treatment. Currently, inactivated vaccines against viruses, such as influenza virus, poliovirus, and hepatitis A virus, are available. VaxigripTetra (Sanofi Pasteur, Lyon, France) is a quadrivalent inactivated influenza vaccine that has been available since 2016. The vaccine contains both A strains (H1N1 and H3N2) and B strains (Victoria and Yamagata) and has been shown to be efficacious and safe in both children and pregnant women [8,9]. Multiple inactivated hepatitis A vaccines, such as VAQTA (Merck, Branchburg, NJ, USA), AVAXIM (Sanofi Pasteur), HAVRIX (GSK, Brentford, UK), and Epaxal (Janssen Biotech Inc., Horsham, PA, USA), are currently available. While hepatitis A vaccinations usually consist of a two-dose schedule, Ott and Wiersma [10] have shown that a single dose of inactivated hepatitis A vaccines can induce anti-hepatitis A virus antibodies that persist for approximately 11 years. In terms of protection against polio, the Centers for Disease Control and Prevention recommends four doses of polio vaccines at specified ages. The inactivated poliovirus vaccine is currently the only vaccine against polio that is administered in the United States, as the oral poliovirus vaccine is associated with a risk of vaccine-derived poliovirus [11]. IPOL (Sanofi Pasteur) is an inactivated vaccine manufactured from three types (1, 2, and 3) of poliovirus that have been shown to cause poliomyelitis.
CoronaVac (Sinovac Life Sciences Co., Ltd., Beijing, China) is an inactivated whole-virus vaccine that was initially approved for emergency use in China, but further studies were deemed necessary to determine the durability of the elicited immune response [12]. Results from a phase III clinical trial in Turkey revealed an 83.5% efficacy 14 days or more after the administration of the second dose of CoronaVac [13]. BBIBP-CorV (Sinopharm, Beijing, China) is another inactivated COVID-19 vaccine that is administered as a two-dose immunization [14]. Phase I/II trial results have shown that this inactivated vaccine is safe and well tolerated, in addition to inducing robust humoral responses [15]. High titers of neutralizing antibodies were reported to be induced in participants vaccinated with inactivated whole-virus vaccines [16]. In terms of vaccine efficacy against VOCs, a 59% vaccine efficacy against infection with the delta variant was reported following a two-dose vaccination with CoronaVac [17].
Unfortunately, technical challenges exist with inactivated whole-virus vaccines, especially in terms of the risk of disease outbreak and the inactivation process that can potentially damage antigens and result in suboptimal immunogenicity. There is also a biosafety level 3 requirement to manufacture inactivated whole-virus vaccines. Furthermore, inactivated vaccines are not known to activate cellular immune responses, and no T cell responses were reported following vaccination with CoronaVac [12]. Therefore, adjuvants and multiple booster doses are crucial to enhance the immune response elicited by inactivated vaccines, and further studies are needed to determine the effect of booster doses on inactivated whole-virus COVID-19 vaccines.
Advances have allowed the genetic manipulation of viruses into suitable vectors that can deliver genetic material. The viruses used as vaccine vectors must be harmless and prompt the target cells to produce antigens that can activate the immune response without causing disease. Among the various viruses, adenoviruses have been the most studied and have shown potential. Adenoviral vectors are built by replacing the viral genes involved in replication with a gene of choice. Most adenoviral vectors are constructed by deleting the E1 and E3 genes, which are involved in viral replication and modulation of the host immune response, respectively [18]. Furthermore, adenoviral vectors are commonly used platforms in cancer gene therapy, which employs adenoviruses engineered to selectively replicate in and kill tumor cells [19]. Antigens delivered by adenoviral vectors after a single immunization have been shown to induce both cellular and humoral immunity, with the second immunization mounting a long-lasting immune response [20,21]. In designing adenovirus vaccine vectors, it is important to select adenovirus serotypes that do not elicit an immune response in humans from preexisting immunity.
Adenoviral vector COVID-19 vaccines exploit adenoviruses to create viral vectors carrying DNA sequences encoding the full-length S protein of SARS-CoV-2. Since the S protein binds to ACE2 for cellular entry, vaccines that produce antibodies able to bind the S protein are expected to neutralize the viral infection [22]. ChAdOX1, AstraZeneca’s COVID-19 vaccine (AstraZeneca, Cambridge, UK) uses a recombinant adenovirus derived from chimpanzees to produce adenoviral vectors carrying the full-length S protein sequence [23]. ChAdOX1 was shown to induce strong cellular immunity, especially in terms of increased effector T cell responses specific to the S protein. A second dose was administered 8 to 12 weeks after the first dose is the recommended vaccination schedule set by the WHO. A study has shown that increasing the interval between primary and booster doses of ChAdOX1 beyond 12 weeks resulted in antibody titers that were higher than those mounted by a second dose administered within 6 weeks of the initial vaccination [24].
Ad26.COV2.S, Janssen Biotech’s COVID-19 vaccine is an S protein-based adenoviral serotype 26 vector vaccine that elicited strong Th1-skewed cellular immune responses during clinical trials [25]. The Janssen vaccine expresses the prefusion form of the S antigen that has undergone stabilizing substitutions [26]. Gam-COVID-Vac (also known as Sputnik V), an adenovirus vector vaccine produced by the Russian Gamaleya Research Institute of Epidemiology and Microbiology, is distinct from the previously mentioned vaccines in terms of its heterologous prime-boost approach. Gam-COVID-Vac utilizes two different adenoviral vectors, Ad26 and Ad5, which are administered individually 21 days apart [27]. CELLID (Seoul, Korea) has also developed AdCLD-CoV19, an adenoviral vector vaccine based on the human Ad type 5/35 vector containing the gene for the S protein of SARS-CoV2, which has been approved for phase I/II trials in the Republic of Korea.
To circumvent the challenges associated with preexisting immunity against certain human adenovirus serotypes, prime-boost regimens, such as those used for Sputnik V, have mainly relied on longer intervals (>12 weeks) or utilization of different serotypes. However, further studies that utilize a combination of different strategies are needed to improve adenoviral vector vaccines.
Prior to the COVID-19 pandemic, nucleic acid-based vaccine candidates against diseases were unable to progress beyond clinical trials. Nucleic acid-based vaccines have the advantage of a shorter development period following sequence selection when compared to virus- or protein-based vaccines. Vaccine technologies utilized by nucleic acid-based platforms also take advantage of nanoparticles in terms of their small size and ability to enter cells and deliver nucleic acids via DNA or messenger RNA (mRNA) vaccines. Lipid nanoparticles can encapsulate genomic materials that carry antigen-encoding sequences.
mRNA-based vaccines that have been approved for use include the Pfizer-BioNTech COVID-19 vaccine (BNT162b2; Pfizer Inc., New York, NY, USA) and Moderna COVID-19 vaccine (mRNA-1273; Moderna Inc., Cambridge, MA, USA). Both rely on the viral genomic sequence encoding the subunits of the S protein of SARS-CoV-2.
The Pfizer-BioNTech COVID-19 vaccine, or Comirnaty, consists of a lipid nanoparticle that encapsulates mRNA encoding a modified, full-length SARS-CoV-2 S protein that has been mutated to maintain a prefusion conformation [30,31]. The vaccine requires two doses to be administered 21 days apart, and studies have reported a 95% efficacy, with protection being observed within 12 days after the first dose. A 6-month follow-up study on the safety profile and need for booster dosing demonstrated a decline in vaccine-mediated protection, with the vaccine efficacy waning approximately 6% every 2 months following the second dose in participants aged 12 years and older [32]. Consequently, a single booster dose of Comirnaty following the primary series has been shown to increase neutralizing antibody titers.
In addition, T cell responses have been shown to be important in controlling SARS-CoV-2 infections, and the prime-boost vaccination regimen with Comirnaty was reported to induce strong Th1-biased T cell responses consisting of high levels of interferon gamma and interleukin-2 [33]. With the recent emergence of the omicron variant, two doses of Comirnaty were reported by Pfizer to confer protection against any severe disease, although a booster dose is recommended. Since the majority of the epitopes on the S protein of the omicron variant are predicted to maintain their ability for human leukocyte antigen-epitope binding, vaccines such as Comirnaty that focus on the S protein are expected to elicit a sufficiently robust T cell immunity against VOCs such as omicron [34-36]. A study on the effectiveness of Pfizer’s Comirnaty and AstraZeneca’s ChAdOX1 against the delta (B.1.617.2) variant demonstrated that the efficacy of both vaccines was lower for the delta variant than for the alpha (B.1.1.7) variant [37]. Furthermore, a heterologous prime-boost vaccination with ChAdOX1 (prime) and Comirnaty (boost) was reported to induce robust humoral and cellular immune responses with T cells that were reactive to variants, including alpha, beta, gamma, and delta [38]. Recent findings have shown that a booster shot with Comirnaty was associated with reduced rates of infection and severe illness across different age groups [39].
The Moderna COVID-19 vaccine (mRNA-1273) contains mRNA encoding the prefusion form of the S antigen with two mutations at amino acids 986 and 987 to stabilize the S protein in its prefusion conformation [31]. The Moderna COVID-19 vaccine schedule consists of a two-dose series separated by 28 days. Both of the previously mentioned mRNA vaccines do not include the use of an adjuvant, as the RNA and lipids themselves have been reported to have adjuvant properties [40]. High levels of neutralizing antibodies and a Th1-skewed T cell response were reported following vaccination with mRNA-1273, with efficacy of approximately 93% for preventing COVID-19, while a higher vaccine efficacy of 98% was observed for preventing severe COVID-19 starting 14 days after the second dose [41,42].
A comparative study on the vaccine efficacy of Moderna’s mRNA-1273, Pfizer-BioNTech’s Comirnaty, and Janssen’s Ad26.COV2.S revealed that the two mRNA vaccines (mRNA-1273 and Comirnaty) had higher vaccine efficacy and induced higher postvaccination anti-SARS-CoV-2 antibody levels than Janssen’s adenoviral vector vaccine (Ad26,COV2.S) in healthy adults [43].
Evaluation of the safety profiles revealed that adverse events, while rare, were associated with the mRNA vaccines of both Moderna and Pfizer-BioNTech. Multiple cases of myocarditis were reported following vaccination, with the highest risk observed in young men between the ages of 20 and 34 years [44,45]. In addition to myocarditis, Bell’s palsy after vaccination with either mRNA vaccine has been reported [46-48].
DNA vaccines have already been integrated into veterinary practice to treat diseases, including tuberculosis [49], avian influenza [50], and rabies [51]. Several vaccine candidates utilizing DNA-based platforms are currently undergoing clinical trials. Genexine’s GX-19 (Genexine, Seongnam, Korea), which contains genes encoding both the S and N proteins, and GeneOne Life Science’s GLS-5310 (GeneOne Life Science, Seoul, Korea) are DNA vaccines that have been approved for phase I and phase IIa clinical trials in the Republic of Korea. Moreover, the International Vaccine Institute (Seoul, Korea) has collaborated with INOVIO Pharmaceuticals (Plymouth Meeting, PA, USA) to advance clinical trials of INO-4800, a DNA vaccine that has been shown to induce cellular and humoral immune responses after the second immunization [52].
While mRNA can be directly translated once inside the cell, DNA must undergo nuclear translocation prior to mRNA being transcribed and exported to the cytoplasm for translation. As a result, mRNA has a higher translation efficiency than DNA when transfected. However, DNA is more stable than mRNA, and expression of the latter is shorter-lived. These type-specific pros and cons of nucleic acids highlight the importance of considering both stability and translation efficiency in terms of developing a vaccine that can produce effective antigens capable of mounting an immune response.
Vaccine technologies based on protein subunits are also viable vaccine candidates for protection against SARS-CoV-2 infection. Novavax’s recombinant SARS-CoV-2 S protein nanoparticle vaccine, NVX-CoV2373 (Novavax, Gaithersburg, MD, USA), includes the prefusion form of the full-length S protein that has been modified for stabilization and resistance to cleavage. The vaccine is administered with Matrix-M adjuvant (Novavax), which has previously been shown to enhance immunogenicity of the influenza vaccine [53-55]. Two doses of NVX-CoV2373 were reported to confer approximately 89% protection against SARS-CoV-2 infection [56]. Subunit vaccines can also include adjuvants to boost immune responses by stimulating the desired receptors responsible for sensing pathogens or danger signals [57]. SK Bioscience’s recombinant protein nanoparticle vaccine candidate GBP510 (SK Bioscience, Seongnam, Korea), which contains alum as an adjuvant, and EuCorVac-19, a recombinant protein vaccine manufactured by EuBiologics (Seoul, Korea), are both currently undergoing phase I/II trials in the Republic of Korea.
Despite the long strides that have been made by the fast-paced development, emergency approval, and administration of the previously mentioned vaccines, the variability in host immune responses, which results in patients who range from asymptomatic to critically ill, remains a difficult obstacle that requires long-term follow-up studies postvaccination.
Although multiple COVID-19 vaccines are now available across the globe, we still face several challenges concerning long-term vaccine efficacy, as well as effectiveness against present and future variants. It is essential to plan for the development of modified vaccines that could protect against vaccine-resistant variants, as we are now witnessing the emergence of VOCs that have increased the transmissibility and virulence of COVID-19. Therefore, the combined use of the diverse platforms for COVID-19 vaccines that are now available will aid in the development of vaccines against current and future variants.
While the global morbidity and mortality caused by COVID-19 emphasize the need for vaccination, adverse events remain a risk associated with the currently available vaccines. While most side effects following vaccination are mild, such as headache and pain/soreness at the injection site, severe adverse events such as myocarditis and thrombocytopenia have also been reported, particularly in young women and men. In the future, we need to focus on developing novel vaccine platforms that are associated with lower risks of adverse events and increasing our efforts toward establishing a universal coronavirus vaccine that can confer broad protection against a diverse number of coronaviruses, including all variants, to increase our preparedness for future pandemics.
Notes
References
1. World Health Organization (WHO). WHO coronavirus (COVID-19) dashboard [Internet]. Geneva: WHO;2021. [cited 2021 Dec 14]. https://covid19.who.int/.
2. Stadler K, Masignani V, Eickmann M, Becker S, Abrignani S, Klenk HD, et al. SARS: beginning to understand a new virus. Nat Rev Microbiol. 2003; 1:209–18.
3. Janeway Jr CA, Travers P, Walport M, Shlomchik MJ. The distribution and functions of immunoglobulin isotypes. In : Janeway Jr CA, Travers P, Walport M, Shlomchik MJ, editors. Immunobiology: the immune system in health and disease. 5th ed. New York: Garland Science;2001.
4. World Health Organization (WHO). Tracking SARS-CoV-2 variants [Internet]. Geneva: WHO;2021. [cited 2021 Dec 14]. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/.
5. World Health Organization (WHO). Weekly epidemiological update on COVID-19-25 [Internet]. Geneva: WHO;2022. [cited 2022 Jan 17]. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---25-january-2022.
6. Centers for Disease Control and Prevention (CDC). COVID data tracker: variant proportions [Internet]. Atlanta: CDC;2022. [cited 2022 Jan 17]. https://covid.cdc.gov/covid-data-tracker/#monitoring-varaint-heading.
7. Beatty AL, Peyser ND, Butcher XE, Cocohoba JM, Lin F, Olgin JE, et al. Analysis of COVID-19 vaccine type and adverse effects following vaccination. JAMA Netw Open. 2021; 4:e2140364.
8. Bansal A, Trieu MC, Mohn KGI, Cox RJ. Safety, immunogenicity, efficacy and effectiveness of inactivated influenza vaccines in healthy pregnant women and children under 5 years: an evidence-based clinical review. Front Immunol. 2021; 12:744774.
9. Pepin S, Dupuy M, Borja-Tabora CF, Montellano M, Bravo L, Santos J, et al. Efficacy, immunogenicity, and safety of a quadrivalent inactivated influenza vaccine in children aged 6-35 months: a multi-season randomised placebo-controlled trial in the Northern and Southern hemispheres. Vaccine. 2019; 37:1876–84.
10. Ott JJ, Wiersma ST. Single-dose administration of inactivated hepatitis A vaccination in the context of hepatitis A vaccine recommendations. Int J Infect Dis. 2013; 17:e939–44.
11. Centers for Disease Control and Prevention (CDC). Polio vaccination [Internet]. Atlanta: CDC;2018. [cited 2022 Jan 17]. https://www.cdc.gov/vaccines/vpd/polio/index.html.
12. Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021; 21:181–92.
13. Tanriover MD, Doğanay HL, Akova M, Güner HR, Azap A, Akhan S, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021; 398:213–22.
14. Wang H, Zhang Y, Huang B, Deng W, Quan Y, Wang W, et al. Development of an inactivated vaccine candidate, BBIBP-CorV, with potent protection against SARS-CoV-2. Cell. 2020; 182:713–21.
15. Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021; 21:39–51.
16. Guo W, Duan K, Zhang Y, Yuan Z, Zhang YB, Wang Z, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18 years or older: a randomized, double-blind, placebo-controlled, phase 1/2 trial. EClinicalMedicine. 2021; 38:101010.
17. Li XN, Huang Y, Wang W, Jing QL, Zhang CH, Qin PZ, et al. Effectiveness of inactivated SARS-CoV-2 vaccines against the Delta variant infection in Guangzhou: a test-negative case-control real-world study. Emerg Microbes Infect. 2021; 10:1751–9.
18. Russell WC. Adenoviruses: update on structure and function. J Gen Virol. 2009; 90(Pt 1):1–20.
19. Buller RE, Runnebaum IB, Karlan BY, Horowitz JA, Shahin M, Buekers T, et al. A phase I/II trial of rAd/p53 (SCH 58500) gene replacement in recurrent ovarian cancer. Cancer Gene Ther. 2002; 9:553–66.
20. Tatsis N, Ertl HC. Adenoviruses as vaccine vectors. Mol Ther. 2004; 10:616–29.
21. Dolzhikova IV, Zubkova OV, Tukhvatulin AI, Dzharullaeva AS, Tukhvatulina NM, Shcheblyakov DV, et al. Safety and immunogenicity of GamEvac-Combi, a heterologous VSV- and Ad5-vectored Ebola vaccine: an open phase I/II trial in healthy adults in Russia. Hum Vaccin Immunother. 2017; 13:613–20.
22. Astuti I. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): an overview of viral structure and host response. Diabetes Metab Syndr. 2020; 14:407–12.
23. Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020; 396:467–78.
24. Voysey M, Costa Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021; 397:881–91.
25. Sadoff J, Le Gars M, Shukarev G, Heerwegh D, Truyers C, de Groot AM, et al. Interim results of a phase 1-2a trial of Ad26.COV2.S COVID-19 vaccine. N Engl J Med. 2021; 384:1824–35.
26. Bos R, Rutten L, van der Lubbe JE, Bakkers MJ, Hardenberg G, Wegmann F, et al. Ad26 vector-based COVID-19 vaccine encoding a prefusion-stabilized SARS-CoV-2 Spike immunogen induces potent humoral and cellular immune responses. NPJ Vaccines. 2020; 5:91.
27. Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021; 397:671–81.
28. Eichinger S, Warkentin TE, Greinacher A. Thrombotic thrombocytopenia after ChAdOx1 nCoV-19 vaccination: reply. N Engl J Med. 2021; 385:e11.
29. Health Alert Network; Centers for Disease Control and Prevention (CDC). Emergency preparedness and response: cases of cerebral venous sinus thrombosis with thrombocytopenia after receipt of the Johnson & Johnson COVID-19 vaccine [Internet]. Atlanta: CDC;2021. [cited 2022 Jan 17]. https://emergency.cdc.gov/han/2021/han00442.asp.
30. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020; 383:2603–15.
31. Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020; 367:1260–3.
32. Thomas SJ, Moreira ED Jr, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine through 6 months. N Engl J Med. 2021; 385:1761–73.
33. Sahin U, Muik A, Vogler I, Derhovanessian E, Kranz LM, Vormehr M, et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature. 2021; 595:572–7.
34. Ahmed SF, Quadeer AA, McKay MR. SARS-CoV-2 T cell responses elicited by COVID-19 vaccines or infection are expected to remain robust against Omicron. Viruses. 2022; 14:79.
35. Tarke A, Sidney J, Methot N, Yu ED, Zhang Y, Dan JM, et al. Impact of SARS-CoV-2 variants on the total CD4+ and CD8+ T cell reactivity in infected or vaccinated individuals. Cell Rep Med. 2021; 2:100355.
36. Stanojevic M, Geiger A, Ostermeier B, Sohai D, Lazarski C, Lang H, et al. Spike-directed vaccination elicits robust spike-specific T-cell response, including to mutant strains. Cytotherapy. 2022; 24:10–5.
37. Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021; 385:585–94.
38. Groß R, Zanoni M, Seidel A, Conzelmann C, Gilg A, Krnavek D, et al. Heterologous ChAdOx1 nCoV-19 and BNT162b2 prime-boost vaccination elicits potent neutralizing antibody responses and T cell reactivity against prevalent SARS-CoV-2 variants. EBioMedicine. 2022; 75:103761.
39. Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Freedman L, Alroy-Preis S, et al. Protection against COVID-19 by BNT162b2 booster across age groups. N Engl J Med. 2021; 385:2421–30.
40. Perrie Y, Crofts F, Devitt A, Griffiths HR, Kastner E, Nadella V. Designing liposomal adjuvants for the next generation of vaccines. Adv Drug Deliv Rev. 2016; 99(Pt A):85–96.
41. Anderson EJ, Rouphael NG, Widge AT, Jackson LA, Roberts PC, Makhene M, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020; 383:2427–38.
42. El Sahly HM, Baden LR, Essink B, Doblecki-Lewis S, Martin JM, Anderson EJ, et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med. 2021; 385:1774–85.
43. Self WH, Tenforde MW, Rhoads JP, Gaglani M, Ginde AA, Douin DJ, et al. Comparative effectiveness of moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) vaccines in preventing COVID-19 hospitalizations among adults without immunocompromising conditions: United States, March-August 2021. MMWR Morb Mortal Wkly Rep. 2021; 70:1337–43.
44. Castiello T, Georgiopoulos G, Finocchiaro G, Claudia M, Gianatti A, Delialis D, et al. COVID-19 and myocarditis: a systematic review and overview of current challenges. Heart Fail Rev. 2022; 27:251–61.
45. Barda N, Dagan N, Ben-Shlomo Y, Kepten E, Waxman J, Ohana R, et al. Safety of the BNT162b2 mRNA COVID-19 vaccine in a nationwide setting. N Engl J Med. 2021; 385:1078–90.
46. Colella G, Orlandi M, Cirillo N. Bell’s palsy following COVID-19 vaccination. J Neurol. 2021; 268:3589–91.
47. Repajic M, Lai XL, Xu P, Liu A. Bell’s Palsy after second dose of Pfizer COVID-19 vaccination in a patient with history of recurrent Bell’s palsy. Brain Behav Immun Health. 2021; 13:100217.
48. Ozonoff A, Nanishi E, Levy O. Bell’s palsy and SARS-CoV-2 vaccines. Lancet Infect Dis. 2021; 21:450–2.
49. Teixeira FM, Teixeira HC, Ferreira AP, Rodrigues MF, Azevedo V, Macedo GC, et al. DNA vaccine using Mycobacterium bovis Ag85B antigen induces partial protection against experimental infection in BALB/c mice. Clin Vaccine Immunol. 2006; 13:930–5.
50. Kodihalli S, Kobasa DL, Webster RG. Strategies for inducing protection against avian influenza A virus subtypes with DNA vaccines. Vaccine. 2000; 18:2592–9.
51. Rai N, Kaushik P, Rai A. Development of rabies DNA vaccine using a recombinant plasmid. Acta Virol. 2005; 49:207–10.
52. Tebas P, Yang S, Boyer JD, Reuschel EL, Patel A, Christensen-Quick A, et al. Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: a preliminary report of an open-label, Phase 1 clinical trial. EClinicalMedicine. 2021; 31:100689.
53. Pallesen J, Wang N, Corbett KS, Wrapp D, Kirchdoerfer RN, Turner HL, et al. Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen. Proc Natl Acad Sci U S A. 2017; 114:E7348–57.
54. Magnusson SE, Altenburg AF, Bengtsson KL, Bosman F, de Vries RD, Rimmelzwaan GF, et al. Matrix-M™ adjuvant enhances immunogenicity of both protein- and modified vaccinia virus Ankara-based influenza vaccines in mice. Immunol Res. 2018; 66:224–33.
55. Reimer JM, Karlsson KH, Lövgren-Bengtsson K, Magnusson SE, Fuentes A, Stertman L. Matrix-M™ adjuvant induces local recruitment, activation and maturation of central immune cells in absence of antigen. PLoS One. 2012; 7:e41451.
56. Heath PT, Galiza EP, Baxter DN, Boffito M, Browne D, Burns F, et al. Safety and efficacy of NVX-CoV2373 COVID-19 vaccine. N Engl J Med. 2021; 385:1172–83.
57. Arunachalam PS, Walls AC, Golden N, Atyeo C, Fischinger S, Li C, et al. Adjuvanting a subunit COVID-19 vaccine to induce protective immunity. Nature. 2021; 594:253–8.
Fig. 1.
Different strategies to develop coronavirus disease 2019 (COVID-19) vaccines. Classical and next-generation platform-based vaccines that have been developed as a countermeasure to the COVID-19 pandemic are shown. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; mRNA, messenger RNA.
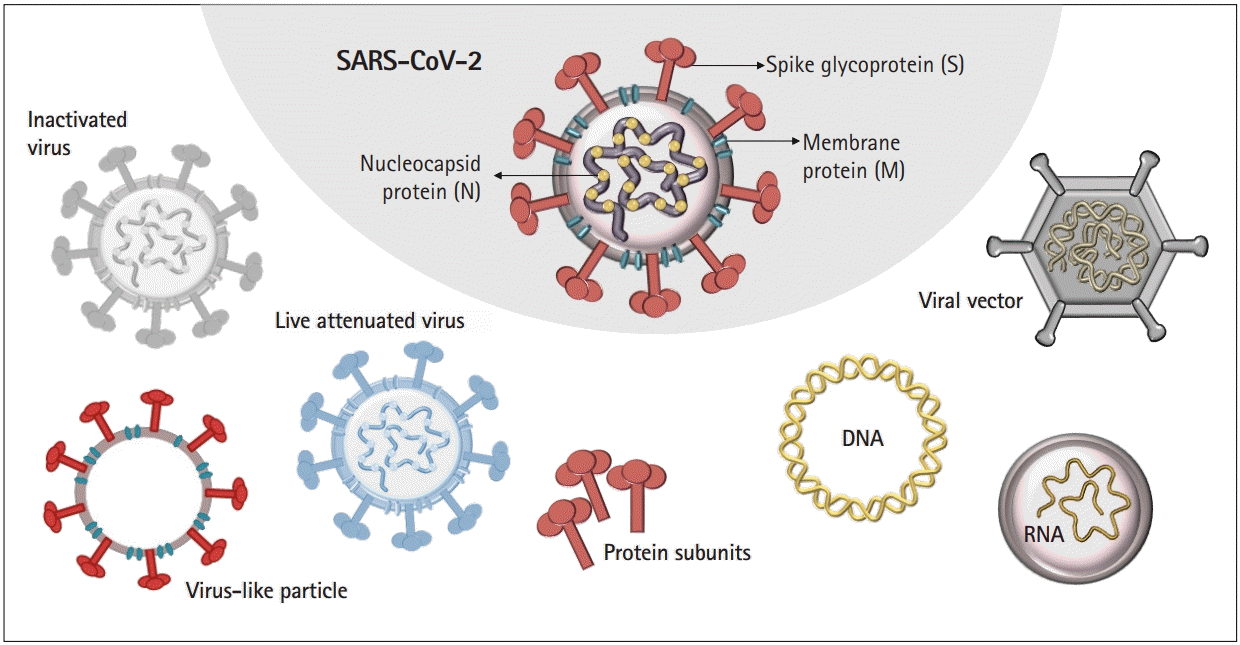
Table 1.
Advantages and disadvantages outlined for each vaccine platform




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download