1. Del Zoppo GJ, Saver JL, Jauch EC, Adams HP Jr. American Heart Association Stroke Council. Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association. Stroke. 2009; 40(8):2945–2948. PMID:
19478221.
2. Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008; 359(13):1317–1329. PMID:
18815396.
3. Bazan HA, Zea N, Jennings B, Smith TA, Vidal G, Sternbergh WC 3rd. Urgent carotid intervention is safe after thrombolysis for minor to moderate acute ischemic stroke. J Vasc Surg. 2015; 62(6):1529–1538. PMID:
26412434.
4. Lovett JK, Coull AJ, Rothwell PM. Early risk of recurrence by subtype of ischemic stroke in population-based incidence studies. Neurology. 2004; 62(4):569–573. PMID:
14981172.
5. Linfante I, Llinas RH, Selim M, Chaves C, Kumar S, Parker RA, et al. Clinical and vascular outcome in internal carotid artery versus middle cerebral artery occlusions after intravenous tissue plasminogen activator. Stroke. 2002; 33(8):2066–2071. PMID:
12154264.
6. Molina CA, Montaner J, Arenillas JF, Ribo M, Rubiera M, Alvarez-Sabín J. Differential pattern of tissue plasminogen activator-induced proximal middle cerebral artery recanalization among stroke subtypes. Stroke. 2004; 35(2):486–490. PMID:
14707233.
7. Kim YS, Garami Z, Mikulik R, Molina CA, Alexandrov AV. CLOTBUST Collaborators. Early recanalization rates and clinical outcomes in patients with tandem internal carotid artery/middle cerebral artery occlusion and isolated middle cerebral artery occlusion. Stroke. 2005; 36(4):869–871. PMID:
15746449.
8. Bhatia R, Hill MD, Shobha N, Menon B, Bal S, Kochar P, et al. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real-world experience and a call for action. Stroke. 2010; 41(10):2254–2258. PMID:
20829513.
9. Rubiera M, Ribo M, Delgado-Mederos R, Santamarina E, Delgado P, Montaner J, et al. Tandem internal carotid artery/middle cerebral artery occlusion: an independent predictor of poor outcome after systemic thrombolysis. Stroke. 2006; 37(9):2301–2305. PMID:
16888266.
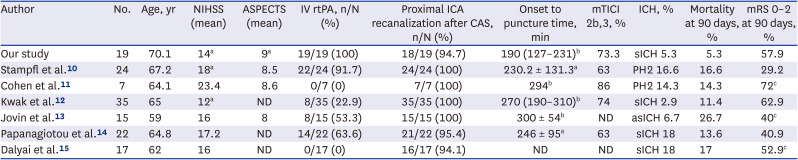
10. Stampfl S, Ringleb PA, Möhlenbruch M, Hametner C, Herweh C, Pham M, et al. Emergency cervical internal carotid artery stenting in combination with intracranial thrombectomy in acute stroke. AJNR Am J Neuroradiol. 2014; 35(4):741–746. PMID:
24157733.
11. Cohen JE, Gomori M, Rajz G, Moscovici S, Leker RR, Rosenberg S, et al. Emergent stent-assisted angioplasty of extracranial internal carotid artery and intracranial stent-based thrombectomy in acute tandem occlusive disease: technical considerations. J Neurointerv Surg. 2013; 5(5):440–446. PMID:
22753268.
12. Kwak HS, Hwang SB, Jin GY, Hippe DS, Chung GH. Predictors of functional outcome after emergency carotid artery stenting and intra-arterial thrombolysis for treatment of acute stroke associated with obstruction of the proximal internal carotid artery and tandem downstream occlusion. AJNR Am J Neuroradiol. 2013; 34(4):841–846. PMID:
23139078.
13. Jovin TG, Gupta R, Uchino K, Jungreis CA, Wechsler LR, Hammer MD, et al. Emergent stenting of extracranial internal carotid artery occlusion in acute stroke has a high revascularization rate. Stroke. 2005; 36(11):2426–2430. PMID:
16224082.
14. Papanagiotou P, Roth C, Walter S, Behnke S, Grunwald IQ, Viera J, et al. Carotid artery stenting in acute stroke. J Am Coll Cardiol. 2011; 58(23):2363–2369. PMID:
22115640.
15. Dalyai RT, Chalouhi N, Singhal S, Jabbour P, Gonzalez LF, Dumont AS, et al. Stent-assisted endovascular recanalization of extracranial internal carotid artery occlusion in acute ischemic stroke. World Neurosurg. 2013; 79(1):143–148. PMID:
23022651.
16. Wechsler LR. Intravenous thrombolytic therapy for acute ischemic stroke. N Engl J Med. 2011; 364(22):2138–2146. PMID:
21631326.
17. Koraen-Smith L, Troëng T, Björck M, Kragsterman B, Wahlgren CM, Kragsterman B, et al. Urgent carotid surgery and stenting may be safe after systemic thrombolysis for stroke. Stroke. 2014; 45(3):776–780. PMID:
24525950.
18. Ratanaprasatporn L, Grossberg JA, Spader HS, Jayaraman MV. Endovascular treatment of acute carotid occlusion. Clin Neurol Neurosurg. 2013; 115(12):2521–2523. PMID:
24239517.
19. Kim DH, Kim B, Jung C, Nam HS, Lee JS, Kim JW, et al. Consensus statements by Korean Society of Interventional Neuroradiology and Korean Stroke Society: hyperacute endovascular treatment workflow to reduce door-to-reperfusion time. J Korean Med Sci. 2018; 33(19):e143. PMID:
29736159.
20. Kang J, Kim SE, Park HK, Cho YJ, Kim JY, Lee KJ, et al. Routing to endovascular treatment of ischemic stroke in Korea: recognition of need for process improvement. J Korean Med Sci. 2020; 35(41):e347. PMID:
33107228.
21. National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995; 333(24):1581–1587. PMID:
7477192.
22. Yamaguchi T, Mori E, Minematsu K, Nakagawara J, Hashi K, Saito I, et al. Alteplase at 0.6 mg/kg for acute ischemic stroke within 3 hours of onset: Japan Alteplase Clinical Trial (J-ACT). Stroke. 2006; 37(7):1810–1815. PMID:
16763187.
23. Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. JAMA. 1995; 274(13):1017–1025. PMID:
7563451.
24. Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver JL, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013; 44(9):2650–2663. PMID:
23920012.
25. Tekieli Ł, Musiałek P, Kabłak-Ziembicka A, Trystuła M, Przewłocki T, Legutko J, et al. Severe, recurrent in-stent carotid restenosis: endovascular approach, risk factors. Results from a prospective academic registry of 2637 consecutive carotid artery stenting procedures (TARGET-CAS). Postepy Kardiol Interwencyjnej. 2019; 15(4):465–471. PMID:
31933663.
26. Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke. 2007; 38(3):967–973. PMID:
17272772.
27. Kimura K, Iguchi Y, Shibazaki K, Aoki J, Uemura J. Early recanalization rate of major occluded brain arteries after intravenous tissue plasminogen activator therapy using serial magnetic resonance angiography studies. Eur Neurol. 2009; 62(5):287–292. PMID:
19713704.
28. Qureshi AI. Endovascular revascularization of symptomatic acute extracranial internal carotid artery occlusion. Stroke. 2005; 36(11):2335–2336. PMID:
16239628.
29. Qureshi AI, Luft AR, Sharma M, Guterman LR, Hopkins LN. Prevention and treatment of thromboembolic and ischemic complications associated with endovascular procedures: part I--Pathophysiological and pharmacological features. Neurosurgery. 2000; 46(6):1344–1359. PMID:
10834640.
30. Le May MR, Labinaz M, Davies RF, Marquis JF, Laramée LA, O’Brien ER, et al. Stenting versus thrombolysis in acute myocardial infarction trial (STAT). J Am Coll Cardiol. 2001; 37(4):985–991. PMID:
11263625.
31. Hussain MA, Alali AS, Mamdani M, Tu JV, Saposnik G, Salata K, et al. Risk of intracranial hemorrhage after carotid artery stenting versus endarterectomy: a population-based study. J Neurosurg. 2018; 129(6):1522–1529. PMID:
29393758.
32. McDonald RJ, Cloft HJ, Kallmes DF. Intracranial hemorrhage is much more common after carotid stenting than after endarterectomy: evidence from the National Inpatient Sample. Stroke. 2011; 42(10):2782–2787. PMID:
21836092.
33. Zinkstok SM, Roos YB. ARTIS investigators. Early administration of aspirin in patients treated with alteplase for acute ischaemic stroke: a randomised controlled trial. Lancet. 2012; 380(9843):731–737. PMID:
22748820.
34. Chandler WL, Alessi MC, Aillaud MF, Henderson P, Vague P, Juhan-Vague I. Clearance of tissue plasminogen activator (TPA) and TPA/plasminogen activator inhibitor type 1 (PAI-1) complex: relationship to elevated TPA antigen in patients with high PAI-1 activity levels. Circulation. 1997; 96(3):761–768. PMID:
9264480.
35. Jeong HG, Kim BJ, Yang MH, Han MK, Bae HJ, Lee SH. Stroke outcomes with use of antithrombotics within 24 hours after recanalization treatment. Neurology. 2016; 87(10):996–1002. PMID:
27521435.
36. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018; 49(3):e46–110. PMID:
29367334.
37. Sallustio F, Koch G, Rocco A, Rossi C, Pampana E, Gandini R, et al. Safety of early carotid artery stenting after systemic thrombolysis: a single center experience. Stroke Res Treat. 2012; 2012:904575. PMID:
21860810.
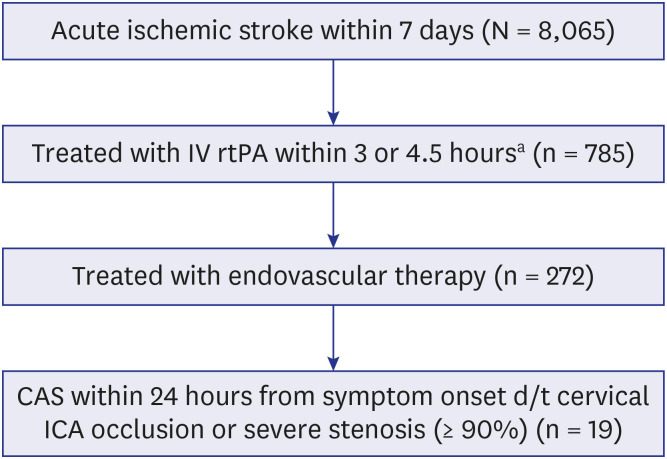
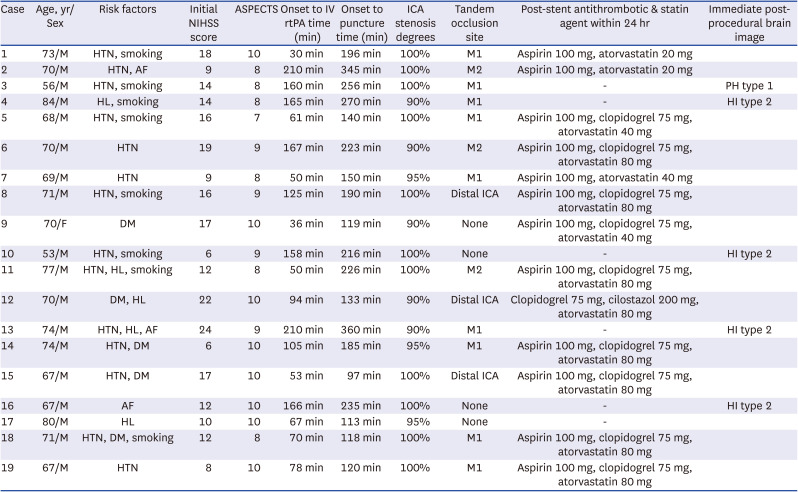
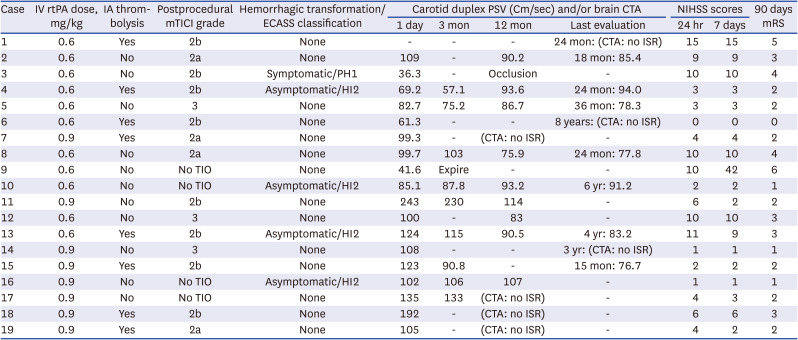
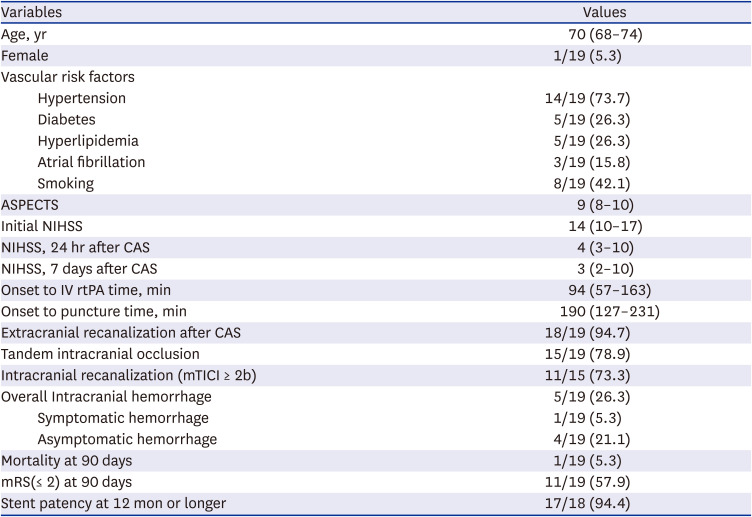




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download