1. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016; 315(8):801–810. PMID:
26903338.
2. Quartin AA, Calonge RO, Schein RM, Crandall LA. Influence of critical illness on physicians’ prognoses for underlying disease: a randomized study using simulated cases. Crit Care Med. 2008; 36(2):462–470. PMID:
18176316.
3. Ince C, Mayeux PR, Nguyen T, Gomez H, Kellum JA, Ospina-Tascón GA, et al. The endothelium in sepsis. Shock. 2016; 45(3):259–270. PMID:
26871664.
4. Acheampong A, Vincent JL. A positive fluid balance is an independent prognostic factor in patients with sepsis. Crit Care. 2015; 19(1):251. PMID:
26073560.
5. Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021; 49(11):e1063–e1143. PMID:
34605781.
6. Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011; 39(2):259–265. PMID:
20975548.
7. Miller WL. Fluid volume overload and congestion in heart failure: time to reconsider pathophysiology and how volume is assessed. Circ Heart Fail. 2016; 9(8):e002922. PMID:
27436837.
8. Duarte K, Monnez JM, Albuisson E, Pitt B, Zannad F, Rossignol P. Prognostic value of estimated plasma volume in heart failure. JACC Heart Fail. 2015; 3(11):886–893. PMID:
26541787.
9. Kobayashi M, Rossignol P, Ferreira JP, Aragão I, Paku Y, Iwasaki Y, et al. Prognostic value of estimated plasma volume in acute heart failure in three cohort studies. Clin Res Cardiol. 2019; 108(5):549–561. PMID:
30341579.
10. Huang CY, Lin TT, Wu YF, Chiang FT, Wu CK. Long-term prognostic value of estimated plasma volume in heart failure with preserved ejection fraction. Sci Rep. 2019; 9(1):14369. PMID:
31591412.
11. Turcato G, Zaboli A, Ciccariello L, Pfeifer N. Estimated plasma volume status (ePVS) could be an easy-to-use clinical tool to determine the risk of sepsis or death in patients with fever. J Crit Care. 2020; 58:106–112. PMID:
32422322.
12. Chouihed T, Rossignol P, Bassand A, Duarte K, Kobayashi M, Jaeger D, et al. Diagnostic and prognostic value of plasma volume status at emergency department admission in dyspneic patients: results from the PARADISE cohort. Clin Res Cardiol. 2019; 108(5):563–573. PMID:
30370469.
13. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987; 40(5):373–383. PMID:
3558716.
14. Pittard MG, Huang SJ, McLean AS, Orde SR. Association of positive fluid balance and mortality in sepsis and septic shock in an Australian cohort. Anaesth Intensive Care. 2017; 45(6):737–743. PMID:
29137585.
15. Hippensteel JA, Uchimido R, Tyler PD, Burke RC, Han X, Zhang F, et al. Intravenous fluid resuscitation is associated with septic endothelial glycocalyx degradation. Crit Care. 2019; 23(1):259. PMID:
31337421.
16. Bateman RM, Sharpe MD, Jagger JE, Ellis CG. Sepsis impairs microvascular autoregulation and delays capillary response within hypoxic capillaries. Crit Care. 2015; 19(1):389. PMID:
26537126.
17. Ince C. The microcirculation is the motor of sepsis. Crit Care. 2005; 9(Suppl 4):S13–S19. PMID:
16168069.
18. Strauss MB, Davis RK, Rosenbaum JD, Rossmeisl EC. Water diuresis produced during recumbency by the intravenous infusion of isotonic saline solution. J Clin Invest. 1951; 30(8):862–868. PMID:
14861307.
19. Fudim M, Miller WL. Calculated estimates of plasma volume in patients with chronic heart failure-comparison with measured volumes. J Card Fail. 2018; 24(9):553–560. PMID:
30098381.
20. Bouchard J, Soroko SB, Chertow GM, Himmelfarb J, Ikizler TA, Paganini EP, et al. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009; 76(4):422–427. PMID:
19436332.
21. Koch T, Geiger S, Ragaller MJ. Monitoring of organ dysfunction in sepsis/systemic inflammatory response syndrome: novel strategies. J Am Soc Nephrol. 2001; 12(Suppl 17):S53–S59. PMID:
11251033.
22. Miranda M, Balarini M, Caixeta D, Bouskela E. Microcirculatory dysfunction in sepsis: pathophysiology, clinical monitoring, and potential therapies. Am J Physiol Heart Circ Physiol. 2016; 311(1):H24–H35. PMID:
27106039.
23. Moreno RP, Metnitz PG, Almeida E, Jordan B, Bauer P, Campos RA, et al. SAPS3--From evaluation of the patient to evaluation of the intensive care unit. Part 2: development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 2005; 31(10):1345–1355. PMID:
16132892.
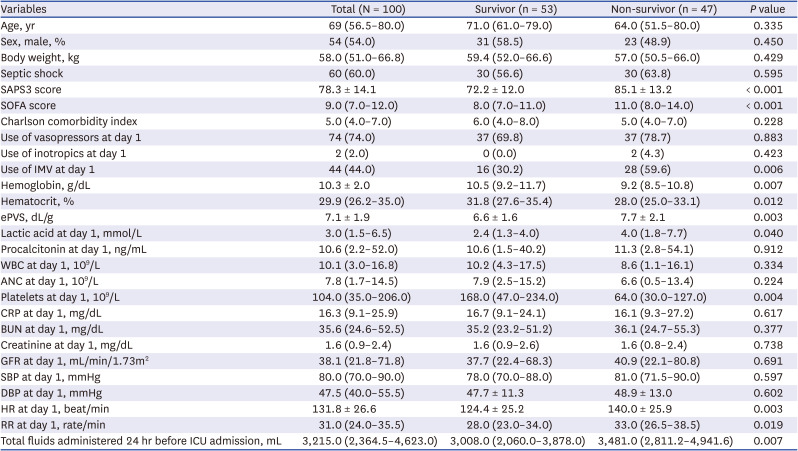
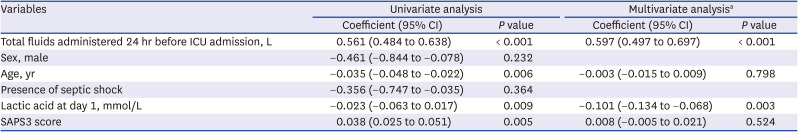
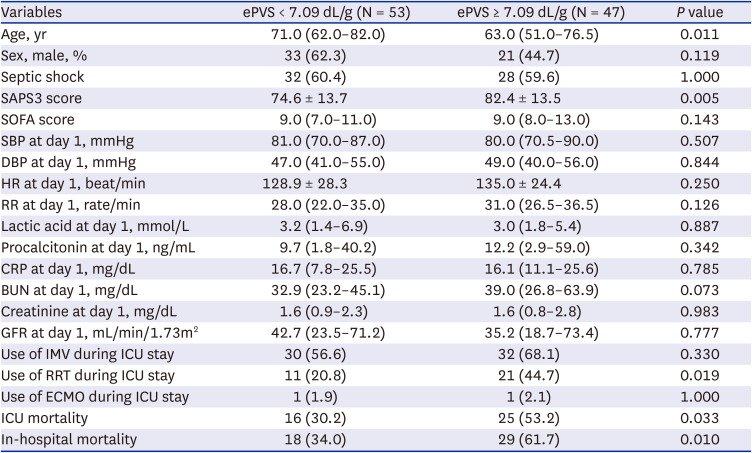
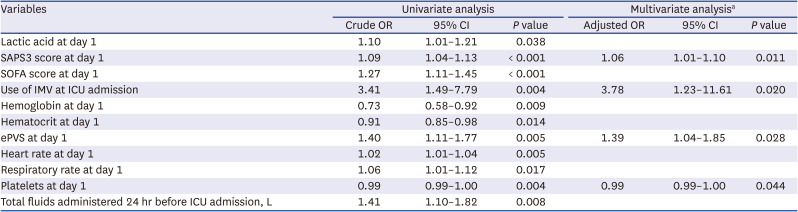




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download