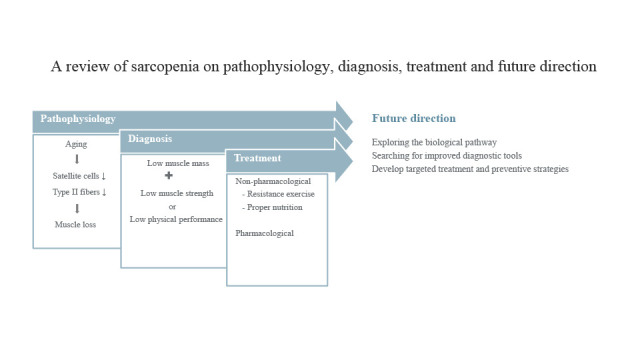
Abstract
Sarcopenia is a progressive and generalized loss of skeletal muscle mass and function. The prevalence of sarcopenia was reported to be up to 29% in older persons in the community healthcare setting. Sarcopenia diagnosis is confirmed by the presence of low muscle mass plus low muscle strength or low physical performance. Sarcopenia management options include non-pharmacological and pharmacological approaches. Non-pharmacological approaches include resistance exercise and adequate nutrition. Of the two, resistance exercise is the standard non-pharmacological treatment approach for sarcopenia with significant positive evidence. Some dietary approaches such as adequate intake of protein, vitamin D, antioxidant nutrients, and long-chain polyunsaturated fatty acid have been shown to have positive effects against sarcopenia. Currently, no specific drugs have been approved by the Food and Drug Administration for the treatment of sarcopenia. However, several agents, including growth hormone, anabolic or androgenic steroids, selective androgenic receptor modulators, protein anabolic agents, appetite stimulants, myostatin inhibitors, activating II receptor drugs, β-receptor blockers, angiotensin-converting enzyme inhibitors, and troponin activators, are recommended and have been shown to have variable efficacy. Future research should focus on sarcopenia biological pathway and improved diagnostic approaches such as biomarkers for early detection, development of consistently pre-eminent treatment methods for severe sarcopenia patients, and establishing sensitive measures for predicting sarcopenia treatment response.
Graphical Abstract
Sarcopenia was derived from the Greek phrase meaning poverty of the flesh and was first described by Rosenberg in 1989.12 Sarcopenia has been defined as an aging-related loss of skeletal muscle mass (SMM) and strength. Several prospective studies have reported that SMM decrease by 6% per decade after mid-life.3 Sarcopenia is thought to involve several pathophysiological processes, such as denervation, mitochondrial dysfunction, and inflammatory and hormonal changes that may lead to adverse health outcomes including falls, functional decline, frailty, and mortality from a decline in lean body mass.456
The prevalence of sarcopenia was reported to be up to 29% in older persons in the community healthcare setting and ranged from 11 to 50% in persons aged 80 years and above.7 Aging disturbs the skeletal muscle homeostasis resulting in an imbalance between anabolic and catabolic processes on the protein production pathway. Cellular changes in sarcopenic muscle are characterized by size and number declines in type II muscle fibers together with intramuscular and intermuscular fat infiltration. In addition, there is a decreased number of satellite cells8910; the main function of satellite cells is to replace and repair damaged muscle fibers. In sarcopenic skeletal muscle, satellite cells’ function may be reduced by alterations of systemic factors that regulate their activity and differentiation such as muscle stem cell niche factors, transforming growth factor-beta (TGF-β), and myogenin. Myogenin is a transcription factor that induces myogenesis in a variety of cell types. TGF-β, myostatin, and bone morphogenetic proteins are the most extensively characterized ligands in terms of the effects on skeletal muscle. Other factors that contribute to muscle loss include neuromuscular junction dysfunction, decreased numbers of motor units,11 inflammation,12 insulin resistance,13 mitochondrial dysfunctions,1415 and oxidative stress.16 Denervation of single muscle fibers is also known to cause a substantial reduction in type II fibers, which are subsequently replaced by type I fibers and fat tissue.
Diagnosis of sarcopenia is based on a proper definition. In 2010, the European Working Group on Sarcopenia in Older People (EWGSOP) proposed three diagnostic criteria for sarcopenia, based on muscle mass, muscle strength, and physical performance.17 Low muscle mass (LMM) is defined by a SMM index of less than 8.90 kg/m2, low muscle strength (LMS) by hand-grip strength below 30 kg in men and 20 kg in women, and low physical performance (LPP) by gait speeds of less than 0.8 m/s. Sarcopenia diagnosis confirmation requires the presence of LMM and LMS or LPP. The EWGSOP classified sarcopenia into three categories: pre-sarcopenia, sarcopenia, and severe sarcopenia based on the presence of LMM and the presence or absence of functional impairment (LMS and LPP). In 2018, the EWGSOP revised their tools and regarded LMS as the primary parameter for sarcopenia diagnosis and muscle strength is currently considered as the most reliable measure of muscle function.5 In the revised guidelines, it was recognized that strength was more effective in predicting adverse outcomes than the mass.18192021 Sarcopenia is probable when LMS is detected; a sarcopenia diagnosis is confirmed by the presence of low muscle quantity or quality. However, use of muscle quality described by the micro- and macroscopic aspects of muscle architecture and composition, as a primary parameter for defining sarcopenia remains a challenge because of the technological limitations.222324 Sarcopenia is considered severe when there is LMS, low muscle quantity or quality, and LPP. In clinical practice, case-finding is warranted when a patient shows the symptoms or signs of sarcopenia (i.e., falling, feeling weak, walking slowly, and difficulties rising from a chair, or weight loss or muscle wasting). In such cases, to confirm diagnosis, the EWGSOP2 recommends the use of the SARC-F questionnaire (a five items tool; Strength, Assistance in walking, Rising from a chair, Climbing stairs, and Falls)25 or the Ishii screening tool (three variables based tool including age, grip strength, and calf circumference).26 In suspected cases, skeletal muscle power has been measured by grip strength using the Jamar or Smedley instruments for arms27 and the chair stand test (sit-to-stand for five times in 30-second intervals) for legs.2829 Muscle quantity or mass can be estimated by a variety of techniques (dual energy X-ray absorptiometry [DXA] or bioelectrical impedance analysis [BIA]), and the results can be adjusted by height or body mass index (BMI).3031 Muscle quantity can be expressed as the total body SMM, appendicular skeletal muscle mass (ASM), or the muscle cross-sectional area of specific muscle groups or body locations (3rd lumbar vertebra3233 or mid-thigh cross-sectional area) by computed tomography or magnetic resonance imaging.3435 Fundamentally, the muscle mass and body size are correlated; individuals with a larger body size normally have larger muscle mass. Thus, when quantifying muscle mass, the absolute SMM or ASM levels can be adjusted for body size in different ways; using height squared (ASM/height2), weight (ASM/weight), or BMI (ASM/BMI).36 Even though calf circumference is not a good measure of muscle mass, it has been shown to predict the performance and survival in older people (at a cutoff of < 31 cm).3738 Physical performance as a multidimensional concept that involves both muscles and central and peripheral nerve function, can be diversely measured by gait speed or the 4-m usual walking speed test (at a cutoff speed of ≤ 0.8 m/s),3940414243 the short physical performance battery (SPPB) (at a cutoff score of ≤ 8 points),17 the time-up and go test (TUG) (at a cutoff time of ≤ 20 seconds),44 and the 400-m walk test.
However, applying Western diagnosis criteria on Asian people may not be adequate, and special considerations should be taken into account. For example, Asians are generally smaller, have more adipose tissue, and more physically active lifestyle. Consequently, the Asian Working Group for Sarcopenia (AWGS) is in the process of developing the consensus for sarcopenia diagnosis through collecting evidence from sarcopenia research studies in Asia.
In 2014, AWGS proposed an algorithm for identifying and diagnosing sarcopenia based on Asian data which was guided by EWGSOP but clearly defined cutoffs for individual diagnostic components.45 AWGS recommends cutoff values for muscle mass measurements (7.0 kg/m2 for men and 5.4 kg/m2 for women using DXA, and 7.0 kg/m2 for men and 5.7 kg/m2 for women using BIA), handgrip strength (< 26 kg for men and < 18 kg for women), and usual gait speed (< 0.8 m/s).
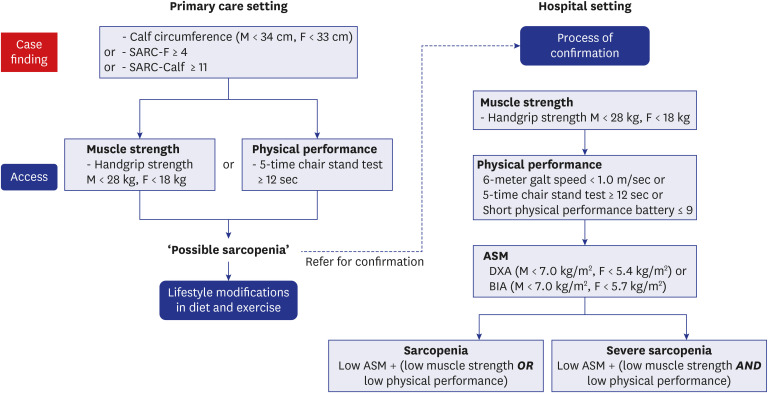
The AWGS in a consensus meeting held in Hong, in May 2019, revised an algorithm for identifying and diagnosing sarcopenia (Fig. 1).46 AWGS 2019 retained the previous sarcopenia definition but revised the diagnostic algorithm, protocols, and some criteria. The cutoffs for LMS in the hand-grip are < 28.0 kg for men and < 18.0 kg for women. The tests for physical performance include the 6-m walk test (with a cutoff speed ≤ 1.0 m/s), TUG (not recommended now), SPPB (with a cutoff score of ≤ 9), and the 5-time chair stand test (with a cutoff time of ≥ 12 seconds).174547 In addition, the AWGS 2019 recommend using either calf circumference (males: < 34 cm, females < 33 cm as the cutoff values) or SARC-F (assessed by the five components: Strength, Assistance in walking, Rising from a chair, Climbing stairs, and Falls, where a score of ≥ 4 is termed as sarcopenia) or the SARC-CalF (composed of the SARC-F plus calf circumference, where a score of ≥ 11 is termed as sarcopenia) for case findings. AWGS 2019 also introduced “possible sarcopenia,” defined as LMS with or without reduced physical performance. In case of low ASM plus LMS or LPP, it is termed as sarcopenia. In case of low ASM, LMS and LPP, it is termed as severe sarcopenia. AWGS 2019 retains the original cutoffs for LMM in a sarcopenia diagnosis: DXA, < 7.0 kg/m2 in men and < 5.4 kg/m2 in women; and BIA, < 7.0 kg/m2 in men and < 5.7 kg/m2 in women.
Sarcopenia is an aging-related muscle mass, strength, and function decline that is associated with variable risk factors. Among them lack of exercise is considered to be the major risk factor; as indicated by aggravated gradual decline in muscle fiber and strength beginning as early as 50 years in patients with sedentary lifestyles.48 Decreases in hormonal concentrations, including growth, sex, and thyroid hormones, and insulin-like growth factor result in the loss of muscle mass and strength. Further, a combination of pro-inflammatory cytokines such as tumor necrosis factor-alpha and interleukin-6 can lead to extreme muscle loss.49505152 Insufficient total protein due to the body’s inability to synthesize protein and inadequate protein intake is contributing factor for severe muscle strength decreases in sarcopenia.53 Aging leads to decreases in motor nerve cells and satellite cells, reducing the response of movement and the recovery of damaged muscle fibers signals.49 Lower birth weight is associated with reduced muscle mass and strength in adults.5455 The options for the management of sarcopenia should be based on an understanding of the pathophysiology and the inclusion of non-pharmacological and pharmacological approaches. Non-pharmacological sarcopenia management approaches include resistance exercise and proper nutrition. Although the efficacy of nutritional intervention without exercise is sarcopenia management is unclear, some evidence showed the benefits of some dietary patterns such as adequate intake of protein, vitamin D, antioxidant nutrients, and long-chain polyunsaturated fatty acid.56 Resistance exercise has been demonstrated as the main no-pharmacological sarcopenia management with significant positive evidence.757 Exercise interventions, especially those based on resistance training, may have a role in improving muscle mass and strength, and physical performance.58 Moreover, resistance exercise is the most effective and affordable sarcopenia progression prevention approach and improves multiple aspects of overall health.59 Notably, at least 3 months or more may be needed to obtain significant improvement in the relevant clinical parameters.7 Further, the impact of resistance exercise on SMM may be augmented by nutritional intervention.59 Leucine-enriched protein supplementation or whey protein is effective in increasing muscle mass and to a lesser extent, muscle function.6061 While vitamin D supplementation increases muscle strength but has no effect on muscle mass,60 a combination of protein and vitamin D can improve functions such as stair climbing, in addition to building muscle mass.6263 Currently, there is no specific drugs have been approved by the Food and Drug Administration for the treatment of sarcopenia. Several agents, including growth hormone, anabolic or androgenic steroids, selective androgenic receptor modulators, protein anabolic agents, appetite stimulants, myostatin inhibitors, activating II receptor drugs, β-receptor blockers, angiotensin converting enzyme inhibitors, and troponin activators, are recommended. However, the treatments have variable efficacy. Growth hormone increases muscle protein synthesis and increases muscle mass but does not improve muscle strength or function.64 Anabolic steroid supplementation effects differed between sexes, with increased weight and lean body mass in men and increased weight, largely due to increased fat mass, in women.65 In both men and women, testosterone supplementation increased muscle strength.66 Notably, the effect of testosterone on muscle mass (more than strength) was higher in men with low serum testosterone levels.67 Herbal supplements such as curcumin, alkaloids, catechins, proanthocyanidin, gingerols, and shogaols showed modest effects on skeletal muscle function.60 Ghrelin and megestrol acetate, which are used as appetite stimulants, can increase body mass and muscle mass.68 Myostatin is produced by muscle and prevent muscle anabolism and satellite cell synthesis.69 It has been associated with reductions in muscle mass. Myostatin effects can be reduced by resistance exercise and myostatin inhibitors which have been shown to have positive effects on muscle mass.69707172 Bimagrumab, a monoclonal antibody against the activin II receptor, increases muscle volume, lean muscle mass, and functional status.63 β-receptor blockers, ACE inhibitors, and troponin activators can have positive effects on muscle mass and grip.737475
Although there are still many information gaps on sarcopenia, future management paths are concentrated on prevention and treatment. Apart from age-related sarcopenia, which is considered as primary sarcopenia, many conditions such as diabetes mellitus7677 and chronic obstructive lung disease7879 can lead to secondary sarcopenia. Analysis and early recognition of the risk factors are important in preventing the sarcopenia progression and complications. Prevention measures include identification of risk factors from a younger age, with a focus on modifying the factors that affect strongly affect risk later in life. Skeletal muscle strength in early adulthood (20–40 years old) might be an important predictor for the development of sarcopenia at later ages. A grip strength of 2.5 standard deviation or more below that of young people is considered low grip strength, and it can be similarly fit the definition of osteoporosis. Exercise is a modifiable risk factor and remains a primary intervention for the prevention and management of sarcopenia. It is therefore important to sensitize young people that healthy lifestyle changes such as physical activities have lifelong benefits for skeletal muscle health. Although the role of nutrition in the treatment of sarcopenia is unclear, optimized diet including adequate protein intake and nutrients is also recommended. Future research should focus on sarcopenia biological pathway and improved diagnostic approaches such as biomarkers for early detection, development of consistently pre-eminent treatment methods for severe sarcopenia patients, and establishing sensitive measures for predicting sarcopenia treatment response. Advanced research and understanding of sarcopenia cellular and molecular mechanisms might play a key role in developing targeted treatment and preventive strategies.80
References
1. Rosenburg I. Summary comments: epidemiological and methodological problems in determining nutritional status of older persons. Am J Clin Nutr. 1989; 50(5):1231–1233.
2. Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997; 127(5):Suppl. 990S–1S. PMID: 9164280.
3. Janssen I. Evolution of sarcopenia research. Appl Physiol Nutr Metab. 2010; 35(5):707–712. PMID: 20962927.
4. Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. J Lab Clin Med. 2001; 137(4):231–243. PMID: 11283518.
5. Lang T, Streeper T, Cawthon P, Baldwin K, Taaffe DR, Harris TB. Sarcopenia: etiology, clinical consequences, intervention, and assessment. Osteoporos Int. 2010; 21(4):543–559. PMID: 19779761.
6. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019; 48(1):16–31. PMID: 30312372.
7. Cruz-Jentoft AJ, Landi F, Schneider SM, Zúñiga C, Arai H, Boirie Y, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing. 2014; 43(6):748–759. PMID: 25241753.
8. Verdijk LB, Snijders T, Drost M, Delhaas T, Kadi F, van Loon LJ. Satellite cells in human skeletal muscle; from birth to old age. Age (Dordr). 2014; 36(2):545–547. PMID: 24122288.
9. Frontera WR, Zayas AR, Rodriguez N. Aging of human muscle: understanding sarcopenia at the single muscle cell level. Phys Med Rehabil Clin N Am. 2012; 23(1):201–207. PMID: 22239884.
10. Ciciliot S, Rossi AC, Dyar KA, Blaauw B, Schiaffino S. Muscle type and fiber type specificity in muscle wasting. Int J Biochem Cell Biol. 2013; 45(10):2191–2199. PMID: 23702032.
11. Edström E, Altun M, Bergman E, Johnson H, Kullberg S, Ramírez-León V, et al. Factors contributing to neuromuscular impairment and sarcopenia during aging. Physiol Behav. 2007; 92(1-2):129–135. PMID: 17585972.
12. Fulop T, Larbi A, Dupuis G, Le Page A, Frost EH, Cohen AA, et al. Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front Immunol. 2018; 8:1960. PMID: 29375577.
13. Walrand S, Zangarelli A, Guillet C, Salles J, Soulier K, Giraudet C, et al. Effect of fast dietary proteins on muscle protein synthesis rate and muscle strength in ad libitum-fed and energy-restricted old rats. Br J Nutr. 2011; 106(11):1683–1690. PMID: 21736767.
14. Huang JH, Hood DA. Age-associated mitochondrial dysfunction in skeletal muscle: Contributing factors and suggestions for long-term interventions. IUBMB Life. 2009; 61(3):201–214. PMID: 19243006.
15. Ferri E, Marzetti E, Calvani R, Picca A, Cesari M, Arosio B. Role of age-related mitochondrial dysfunction in sarcopenia. Int J Mol Sci. 2020; 21(15):5236–5247.
16. Ji LL. Exercise at old age: Does it increase or alleviate oxidative stress? Ann N Y Acad Sci. 2001; 928(1):236–247. PMID: 11795515.
17. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010; 39(4):412–423. PMID: 20392703.
18. Schaap LA, van Schoor NM, Lips P, Visser M. Associations of sarcopenia definitions, and their components, with the incidence of recurrence falling and fractures: the longitudinal aging study in Amsterdam. J Gerontol A Biol Sci Med Sci. 2018; 73(9):1199–1204. PMID: 29300839.
19. Ibrahim K, May C, Patel HP, Baxter M, Sayer AA, Roberts H. A feasibility study of implementing grip strength measurement into routine hospital practice (GRImP): study protocol. Pilot Feasibility Stud. 2016; 2(1):27. PMID: 27965846.
20. Leong DP, Teo KK, Rangarajan S, Lopez-Jaramillo P, Avezum A Jr, Orlandini A, et al. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet. 2015; 386(9990):266–273. PMID: 25982160.
21. Schaap LA, Koster A, Visser M. Adiposity, muscle mass, and muscle strength in relation to functional decline in older persons. Epidemiol Rev. 2013; 35(1):51–65. PMID: 23221972.
22. Buckinx F, Landi F, Cesari M, Fielding RA, Visser M, Engelke K, et al. Pitfalls in the measurement of muscle mass: a need for a reference standard. J Cachexia Sarcopenia Muscle. 2018; 9(2):269–278. PMID: 29349935.
23. Masanés F, Rojano I Luque X, Salvà A, Serra-Rexach JA, Artaza I, Formiga F, et al. Cut-off points for muscle mass-not grip strength or gait speed-determine variations in sarcopenia prevalence. J Nutr Health Aging. 2017; 21(7):825–829. PMID: 28717813.
24. Treviño-Aguirre E, López-Teros T, Gutiérrez-Robledo L, Vandewoude M, Pérez-Zepeda M. Availability and use of dual energy X-ray absorptiometry (DXA) and bio-impedance analysis (BIA) for the evaluation of sarcopenia by Belgian and Latin American geriatricians. J Cachexia Sarcopenia Muscle. 2014; 5(1):79–81. PMID: 24442632.
25. Bahat G, Yilmaz O, Kılıç C, Oren MM, Karan MA. Performance of SARC-F in regard to sarcopenia definitions, muscle mass and functional measures. J Nutr Health Aging. 2018; 22(8):898–903. PMID: 30272090.
26. Ishii S, Tanaka T, Shibasaki K, Ouchi Y, Kikutani T, Higashiguchi T, et al. Development of a simple screening test for sarcopenia in older adults. Geriatr Gerontol Int. 2014; 14(Suppl 1):93–101. PMID: 24450566.
27. Roberts HC, Denison HJ, Martin HJ, Patel HP, Syddall H, Cooper C, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011; 40(4):423–429. PMID: 21624928.
28. Beaudart C, McCloskey E, Bruyère O, Cesari M, Rolland Y, Rizzoli R, et al. Sarcopenia in daily practice: assessment and management. BMC Geriatr. 2016; 16(1):170. PMID: 27716195.
29. Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport. 1999; 70(2):113–119. PMID: 10380242.
30. Cooper C, Fielding R, Visser M, van Loon LJ, Rolland Y, Orwoll E, et al. Tools in the assessment of sarcopenia. Calcif Tissue Int. 2013; 93(3):201–210. PMID: 23842964.
31. Cawthon PM, Peters KW, Shardell MD, McLean RR, Dam TT, Kenny AM, et al. Cutpoints for low appendicular lean mass that identify older adults with clinically significant weakness. J Gerontol A Biol Sci Med Sci. 2014; 69(5):567–575. PMID: 24737559.
32. Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008; 33(5):997–1006. PMID: 18923576.
33. Kim EY, Kim YS, Park I, Ahn HK, Cho EK, Jeong YM. Prognostic significance of CT-determined sarcopenia in patients with small-cell lung cancer. J Thorac Oncol. 2015; 10(12):1795–1799. PMID: 26484630.
34. Lee SJ, Janssen I, Heymsfield SB, Ross R. Relation between whole-body and regional measures of human skeletal muscle. Am J Clin Nutr. 2004; 80(5):1215–1221. PMID: 15531668.
35. Baracos VE, Reiman T, Mourtzakis M, Gioulbasanis I, Antoun S. Body composition in patients with non-small cell lung cancer: a contemporary view of cancer cachexia with the use of computed tomography image analysis. Am J Clin Nutr. 2010; 91(4):1133S–7S. PMID: 20164322.
36. Kim KM, Jang HC, Lim S. Differences among skeletal muscle mass indices derived from height-, weight-, and body mass index-adjusted models in assessing sarcopenia. Korean J Intern Med. 2016; 31(4):643–650. PMID: 27334763.
37. Tosato M, Marzetti E, Cesari M, Savera G, Miller RR, Bernabei R, et al. Measurement of muscle mass in sarcopenia: from imaging to biochemical markers. Aging Clin Exp Res. 2017; 29(1):19–27. PMID: 28176249.
38. Landi F, Onder G, Russo A, Liperoti R, Tosato M, Martone AM, et al. Calf circumference, frailty and physical performance among older adults living in the community. Clin Nutr. 2014; 33(3):539–544. PMID: 23948128.
39. Abellan van Kan G, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009; 13(10):881–889. PMID: 19924348.
40. Peel NM, Kuys SS, Klein K. Gait speed as a measure in geriatric assessment in clinical settings: a systematic review. J Gerontol A Biol Sci Med Sci. 2013; 68(1):39–46. PMID: 22923430.
41. Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait speed and survival in older adults. JAMA. 2011; 305(1):50–58. PMID: 21205966.
42. Maggio M, Ceda GP, Ticinesi A, De Vita F, Gelmini G, Costantino C, et al. Instrumental and non-instrumental evaluation of 4-m walking speed in older individuals. PLoS One. 2016; 11(4):e0153583. PMID: 27077744.
43. Rydwik E, Bergland A, Forsén L, Frändin K. Investigation into the reliability and validity of the measurement of elderly people’s clinical walking speed: a systematic review. Physiother Theory Pract. 2012; 28(3):238–256. PMID: 21929322.
44. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991; 39(2):142–148. PMID: 1991946.
45. Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014; 15(2):95–101. PMID: 24461239.
46. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 consensus updated on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020; 21(3):300–307.e2. PMID: 32033882.
47. Chen LK, Lee WJ, Peng LN, Liu LK, Arai H, Akishita M, et al. Recent advances in sarcopenia research in Asia: 2016 update from the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2016; 17(8):767.e1–767.e7.
48. Faulkner JA, Larkin LM, Claflin DR, Brooks SV. Age-related changes in the structure and function of skeletal muscles. Clin Exp Pharmacol Physiol. 2007; 34(11):1091–1096. PMID: 17880359.
49. Ryall JG, Schertzer JD, Lynch GS. Cellular and molecular mechanisms underlying age-related skeletal muscle wasting and weakness. Biogerontology. 2008; 9(4):213–228. PMID: 18299960.
50. Han JW, Kim DI, Nam HC, Chang UI, Yang JM, Song DS. Association between serum tumor necrosis factor-α and sarcopenia in liver cirrhosis. Clin Mol Hepatol. 2022; 28(2):219–231. PMID: 34281295.
51. Lin B, Bai L, Wang S, Lin H. The association of systemic interleukin 6 and interleukin 10 levels with sarcopenia in elderly patients with chronic obstructive pulmonary disease. Int J Gen Med. 2021; 14:5893–5902. PMID: 34566428.
52. Choi K, Jang HY, Ahn JM, Hwang SH, Chung JW, Choi YS, et al. The association of the serum levels of myostatin, follistatin, and interleukin-6 with sarcopenia, and their impacts on survival in patients with hepatocellular carcinoma. Clin Mol Hepatol. 2020; 26(4):492–505. PMID: 32646201.
53. Marcell TJ. Sarcopenia: causes, consequences, and preventions. J Gerontol A Biol Sci Med Sci. 2003; 58(10):M911–M916. PMID: 14570858.
54. Patel HP, Jameson KA, Syddall HE, Martin HJ, Stewart CE, Cooper C, et al. Developmental influences, muscle morphology, and sarcopenia in community-dwelling older men. J Gerontol A Biol Sci Med Sci. 2012; 67A(1):82–87.
55. Petermann-Rocha F, Chen M, Gray SR, Ho FK, Pell JP, Celis-Morales C. Factors associated with sarcopenia: a cross-sectional analysis using UK Biobank. Maturitas. 2020; 133:60–67. PMID: 32005425.
56. Robinson SM, Reginster JY, Rizzoli R, Shaw SC, Kanis JA, Bautmans I, et al. Does nutrition play a role in the prevention and management of sarcopenia? Clin Nutr. 2018; 37(4):1121–1132. PMID: 28927897.
57. Valenzuela T. Efficacy of progressive resistance training interventions in older adults in nursing homes: a systematic review. J Am Med Dir Assoc. 2012; 13(5):418–428. PMID: 22169509.
58. Suetta C, Andersen JL, Dalgas U, Berget J, Koskinen S, Aagaard P, et al. Resistance training induces qualitative changes in muscle morphology, muscle architecture, and muscle function in elderly postoperative patients. J Appl Physiol (1985). 2008; 105(1):180–186. PMID: 18420714.
59. McKendry J, Currier BS, Lim C, Mcleod JC, Thomas AC, Phillips SM. Nutritional supplements to support resistance exercise in countering the sarcopenia of aging. Nutrients. 2020; 12(7):2057.
60. Gryson C, Ratel S, Rance M, Penando S, Bonhomme C, Le Ruyet P, et al. Four-month course of soluble milk proteins interacts with exercise to improve muscle strength and delay fatigue in elderly participants. J Am Med Dir Assoc. 2014; 15(12):958.e1–958.e9. PMID: 25444576.
61. Martínez-Arnau FM, Fonfría-Vivas R, Buigues C, Castillo Y, Molina P, Hoogland AJ, et al. Effects of leucine administration in sarcopenia: a randomized and placebo-controlled clinical trial. Nutrients. 2020; 12(4):932.
62. Bauer JM, Verlaan S, Bautmans I, Brandt K, Donini LM, Maggio M, et al. Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc. 2015; 16(9):740–747. PMID: 26170041.
63. Yoo JI, Chung HJ, Kim BG, Jung YK, Baek KW, Song MG, et al. Comparative analysis of the association between various serum vitamin D biomarkers and sarcopenia. J Clin Lab Anal. 2021; 35(9):e23946. PMID: 34350631.
64. Sakuma K, Yamaguchi A. Sarcopenia and age-related endocrine function. Int J Endocrinol. 2012; 2012:127362. PMID: 22690213.
65. Meriggioli MN, Roubenoff R. Prospect for pharmacological therapies to treat skeletal muscle dysfunction. Calcif Tissue Int. 2015; 96(3):234–242. PMID: 25363509.
66. Morley JE. Should frailty be treated with testosterone? Aging Male. 2011; 14(1):1–3. PMID: 20670101.
67. Snyder PJ, Bhasin S, Cunningham GR, Matsumoto AM, Stephens-Shields AJ, Cauley JA, et al. Lessons from the testosterone trials. Endocr Rev. 2018; 39(3):369–386. PMID: 29522088.
68. Rondanelli M, Miccono A, Peroni G, Guerriero F, Morazzoni P, Riva A, et al. A systematic review on the effects of botanicals on skeletal muscle health in order to prevent sarcopenia. Evid Based Complement Alternat Med. 2016; 2016:5970367. PMID: 27051451.
69. Argiles JM, Stemmler B. The potential of ghrelin in the treatment of cancer cachexia. Expert Opin Biol Ther. 2013; 13(1):67–76. PMID: 23078025.
70. Morley JE. Pharmacologic options for the treatment of sarcopenia. Calcif Tissue Int. 2016; 98(4):319–333. PMID: 26100650.
71. Jang J, Park S, Kim Y, Jung J, Lee J, Chang Y, et al. Myostatin inhibition-induced increase in muscle mass and strength was amplified by resistance exercise training, and dietary essential amino acids improved muscle quality in mice. Nutrients. 2021; 13(5):1508. PMID: 33947024.
72. Elkina Y, von Haehling S, Anker SD, Springer J. The role of myostatin in muscle wasting: an overview. J Cachexia Sarcopenia Muscle. 2011; 2(3):143–151. PMID: 21966641.
73. Stewart Coats AJ, Ho GF, Prabhash K, von Haehling S, Tilson J, Brown R, et al. Espindolol for the treatment and prevention of cachexia in patients with stage III/IV non-small cell lung cancer or colorectal cancer: a randomized, double-blind, placebo-controlled, international multicentre phase II study (the ACT-ONE trial). J Cachexia Sarcopenia Muscle. 2016; 7(3):355–365. PMID: 27386169.
74. Hutcheon SD, Gillespie ND, Crombie IK, Struthers AD, McMurdo ME. Perindopril improves six minute walking distance in older patients with left ventricular systolic dysfunction: a randomised double blind placebo controlled trial. Heart. 2002; 88(4):373–377. PMID: 12231595.
75. Hwee DT, Kennedy A, Ryans J, Russell AJ, Jia Z, Hinken AC, et al. Fast skeletal muscle troponin activator tirasemtiv increases muscle function and performance in the B6SJL-SOD1G93A ALS mouse model. PLoS One. 2014; 9(5):e96921. PMID: 24805850.
76. Liccini A, Malmstrom TK. Frailty and sarcopenia as predictors of adverse health outcomes in persons with diabetes mellitus. J Am Med Dir Assoc. 2016; 17(9):846–851. PMID: 27569712.
77. Chung SM, Moon JS, Chang MC. Prevalence of sarcopenia and its association with diabetes: a meta-analysis of community-dwelling Asian population. Front Med (Lausanne). 2021; 8:681232. PMID: 34095184.
78. van de Bool C, Rutten EP, van Helvoort A, Franssen FM, Wouters EF, Schols AM. A randomized clinical trial investigating the efficacy of targeted nutrition as adjunct to exercise training in COPD. J Cachexia Sarcopenia Muscle. 2017; 8(5):748–758. PMID: 28608438.
79. Kim SH, Shin MJ, Shin YB, Kim KU. Sarcopenia associated with chronic obstructive pulmonary disease. J Bone Metab. 2019; 26(2):65–74. PMID: 31223602.
80. Dodds RM, Davies K, Granic A, Hollingsworth KG, Warren C, Gorman G, et al. Mitochondrial respiratory chain function and content are preserved in the skeletal muscle of active very old men and women. Exp Gerontol. 2018; 113:80–85. PMID: 30266472.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download