This article has been
cited by other articles in ScienceCentral.
Abstract
Background
The potential for a nosocomial outbreak of coronavirus disease 2019 (COVID-19) from a fully vaccinated individual is largely unknown.
Methods
In October 2021, during the time when the delta variant was dominant, a nosocomial outbreak of COVID-19 occurred in two wards in a tertiary care hospital in Seoul, Korea. We performed airflow investigations and whole-genome sequencing (WGS) of the virus.
Results
The index patient developed symptoms 1 day after admission, and was diagnosed with COVID-19 on day 4 post-admission. He was fully vaccinated (ChAdOx1 nCoV-19) 2 months before the diagnosis. Three inpatients and a caregiver in the same room, two inpatients in an adjacent room, two inpatients in rooms remote from the index room, and one nurse on the ward tested positive. Also, two resident doctors who stayed in an on-call room located on the same ward tested positive (although they had no close contact), as well as a caregiver who stayed on an adjacent ward, and a healthcare worker who had casual contact with this caregiver. Samples from five individuals were available for WGS, and all showed ≤ 1 single-nucleotide polymorphism difference. CCTV footage showed that the index case walked frequently in the corridors of two wards. An airflow study showed that the air from the corridor flowed into the resident on-call room, driven by an air circulator that was always turned on.
Conclusion
Transmission of severe acute respiratory syndrome coronavirus 2 from a fully vaccinated index occurred rapidly via the wards and on-call room. Care must be taken to not use equipment that can change the airflow.
Keywords: SARS-CoV-2, COVID-19, Vaccination, Airborne Transmission
INTRODUCTION
Nosocomial coronavirus disease 2019 (COVID-19) outbreaks are associated with a poor patient prognosis, as well as disruption of medical services due to staff shortages.
1 Because inpatients frequently have co-morbidities or are immunocompromised, they are susceptible to severe COVID-19 infection. Thus, to prevent nosocomial outbreaks of COVID-19, many hospitals use multi-layered preventive strategies such as universal masking, universal reverse transcription polymerase chain reaction testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), screening of patients, resident caregivers, and healthcare workers (HCWs)’ symptoms and epidemiologic links, and vaccination.
Since December 2020, many countries have implemented COVID-19 vaccinations; however, breakthrough infections have occurred due to emergence of the delta and Omicron variant and waning vaccine-induced immunity.
23 Recent report suggest that vaccinated individuals transmit the virus less than non-vaccinated individuals
45; however, a few reports show that fully vaccinated individuals can still cause an outbreak. Recently, we experienced a nosocomial outbreak of COVID-19 on two surgery wards in a tertiary care hospital during the time when the delta variant was predominant. Our experience suggests that fully vaccinated patients may become super-spreaders, and that airborne transmission can be driven by an air circulator.
METHODS
Setting
A COVID-19 outbreak occurred on two surgery wards (wards A and B) at Asan Medical Center, a 2,700-bed tertiary care center in Seoul, Korea, during October 2021. The wards are divided into 22 rooms; five are five-patient rooms, 12 are two-patient rooms, and five are single-patient rooms. The two wards are adjacent to each other (
Fig. 1). At pre-admission, all patients were screened for SARS-CoV-2 using real-time polymerase chain reaction (PCR)s, and then tested again during their stay (day 4, 8, and 14) regardless of symptoms. In addition, resident caregivers took routine SARS-CoV-2 tests before visiting the hospital, and on days 4, 8, and 14 of residency. In addition, they underwent SARS-CoV-2 testing if they showed any symptoms or signs of COVID-19. A universal mask-wearing policy was mandated in the hospital; however, inpatients and caregivers were often unmasked in their rooms, especially when sleeping or eating.
Fig. 1
Floor plan of wards A and ward B. Individuals with coronavirus disease 2019 are indicated by case number.

Response to the outbreak
After the first case of COVID-19 was confirmed on ward A, the patient was admitted to a negative pressure isolation room on a designated COVID-19 ward. All patients, caregivers, and nurses on the ward where the first case stayed, as well as other exposed HCWs, underwent SARS-CoV-2 testing. Inpatients and caregivers who were close contacts were isolated in single rooms. HCWs who were close contacts were quarantined. Epidemiologic investigations were performed. All contacts were interviewed and categorized according to the nature of their activity, duration of exposure, and the personal protective equipment (PPE) worn at the time of exposure. Close contacts were defined as 1) those who were in close proximity (< 6 feet) to the index for at least 15 minutes within 2 days of symptom development, or within 2 days of the date of collection of a positive specimen when the index was asymptomatic; 2) inpatients or guardians who shared the same (multi-patient) room with the index case, or anyone who had a meal with the index case (equivalent to “household” exposure); or 3) contact with the index case when an aerosol-generating event occurred and they were not wearing appropriate PPE (an N95 or FFP2-equivalent respirator, a face shield/goggles, a gown, and gloves).
6 Non-close contacts were defined as those who did not meet the criteria of a close contact, but had possible temporal or spatial contact with the index case. Whenever possible, whole-genome sequencing (WGS) of specimens from the index and secondary cases was conducted to confirm or refute infection. Infections in patients, HCWs, and caregivers with WGS data showing sequences with more than two single-nucleotide polymorphisms were deemed not to be cluster-related.
WGS
To analyze viral genomic sequences from the index sample, viral RNA was extracted using a QIAamp viral RNA Mini Kit (Qiagen, Hilden, Germany). To isolate pure SARS-CoV-2 RNA, human ribosomal RNA was depleted using the NEBNext rRNA Depletion Kit (New England Biolabs, Ipswich, MA, USA). Libraries were prepared using the TruSeq RNA sample prep kit v2 (Illumina, San Diego, CA, USA) protocol. The enriched libraries were quantified using the Kapa Library Quantification Kit (Roche, Basel, Switzerland), and all sequencing was performed using the Miseq platform (Illumina) with Miseq reagent kit v2 (300 cycles) (Illumina). Sequencing analysis was performed using CLC Genomics Workbench 10 (QIAGEN). Base-called reads in FASTQ were trimmed and mapped to a reference sequence (NCBI Reference: NC_045512).
RESULTS
Description of the index case and infection control
On October 19, 2021, a 60-year-old male (case 1) was admitted to hospital with abdominal pain. He had a history of recurrent mechanical ileus after laparoscopic abdominoperineal resection due to anal cancer. After assessment of mechanical ileus, he was admitted to a six-patient room (room No. 35) on ward A. A SARS-CoV-2 PCR test on the day of admission was negative. Three days before admission (October 16), he had been to Dongdaemun market and had a meal in a Chinese restaurant where a large outbreak occurred. On October 20, he developed symptoms, including a dry cough and chills. On hospital day 4 (October 22), a SARS-CoV-2 PCR testing conducted for universal screening purposes was positive (Ct value for the E gene = 21.04). He had received the second dose of the ChAdOx1-nCoV19 vaccine on August 27 (56 days before diagnosis). CCTV footage showed that case 1 had often walked along all corridors of ward A and B for ambulation treatment with mask wearing.
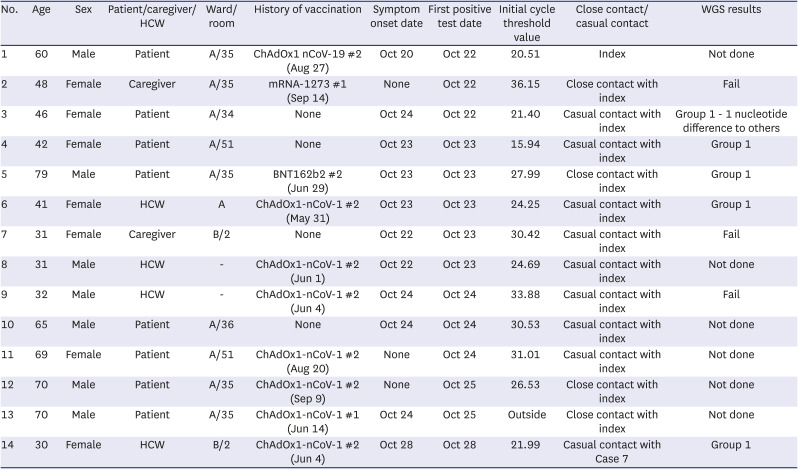
Immediately after identification of case 1, all patients and caregivers on the ward underwent SARS-CoV-2 PCR testing, which was repeated every 3 days until 14 days after the last exposure, and/or as soon as symptoms developed. Four roommates of the index case (cases 2, 5, 12, and 13), two inpatients who stayed next to the index’s room (cases 3 and 10), two inpatients who stayed remotely from the index’s room (cases 4 and 11), and one nurse (case 6) who had cared for cases 4 and 11 tested positive within 3 days after the index diagnosis (
Table 1). In addition, two resident doctors (cases 8 and 9) who stayed in the on-call room (which was located on the same ward) tested positive, although they had no close contact with the index or other cases. Case 4 and 11 were close contact each other as they were roommates in a two-bed room from October 18 to October 23 (
Fig. 1). In addition, case 8 and 9 stayed in on-call room at the same time (≥ 15 minutes) and they stated that they talked each other without mask. Case 7, who was a caregiver, stayed for 5 days on ward B; she was tested for SARS-CoV-2 after developing myalgia on October 23. On October 28, a phlebotomist who took a blood sample from the patients cared for by case 7 also tested positive. The epidemic curve based on the date of symptom onset is shown in
Fig. 2.
Table 1
Line list of individuals with coronavirus disease 2019
|
No. |
Age |
Sex |
Patient/caregiver/HCW |
Ward/room |
History of vaccination |
Symptom onset date |
First positive test date |
Initial cycle threshold value |
Close contact/casual contact |
WGS results |
|
1 |
60 |
Male |
Patient |
A/35 |
ChAdOx1 nCoV-19 #2 (Aug 27) |
Oct 20 |
Oct 22 |
20.51 |
Index |
Not done |
|
2 |
48 |
Female |
Caregiver |
A/35 |
mRNA-1273 #1 (Sep 14) |
None |
Oct 22 |
36.15 |
Close contact with index |
Fail |
|
3 |
46 |
Female |
Patient |
A/34 |
None |
Oct 24 |
Oct 22 |
21.40 |
Casual contact with index |
Group 1 - 1 nucleotide difference to others |
|
4 |
42 |
Female |
Patient |
A/51 |
None |
Oct 23 |
Oct 23 |
15.94 |
Casual contact with index |
Group 1 |
|
5 |
79 |
Male |
Patient |
A/35 |
BNT162b2 #2 (Jun 29) |
Oct 23 |
Oct 23 |
27.99 |
Close contact with index |
Group 1 |
|
6 |
41 |
Female |
HCW |
A |
ChAdOx1-nCoV-1 #2 (May 31) |
Oct 23 |
Oct 23 |
24.25 |
Casual contact with index |
Group 1 |
|
7 |
31 |
Female |
Caregiver |
B/2 |
None |
Oct 22 |
Oct 23 |
30.42 |
Casual contact with index |
Fail |
|
8 |
31 |
Male |
HCW |
- |
ChAdOx1-nCoV-1 #2 (Jun 1) |
Oct 22 |
Oct 23 |
24.69 |
Casual contact with index |
Not done |
|
9 |
32 |
Male |
HCW |
- |
ChAdOx1-nCoV-1 #2 (Jun 4) |
Oct 24 |
Oct 24 |
33.88 |
Casual contact with index |
Fail |
|
10 |
65 |
Male |
Patient |
A/36 |
None |
Oct 24 |
Oct 24 |
30.53 |
Casual contact with index |
Not done |
|
11 |
69 |
Female |
Patient |
A/51 |
ChAdOx1-nCoV-1 #2 (Aug 20) |
None |
Oct 24 |
31.01 |
Casual contact with index |
Not done |
|
12 |
70 |
Male |
Patient |
A/35 |
ChAdOx1-nCoV-1 #2 (Sep 9) |
None |
Oct 25 |
26.53 |
Close contact with index |
Not done |
|
13 |
70 |
Male |
Patient |
A/35 |
ChAdOx1-nCoV-1 #1 (Jun 14) |
Oct 24 |
Oct 25 |
Outside |
Close contact with index |
Not done |
|
14 |
30 |
Female |
HCW |
B/2 |
ChAdOx1-nCoV-1 #2 (Jun 4) |
Oct 28 |
Oct 28 |
21.99 |
Casual contact with Case 7 |
Group 1 |
Fig. 2
Epidemic curve.
HCW = healthcare worker, WGS = whole-genome sequencing.
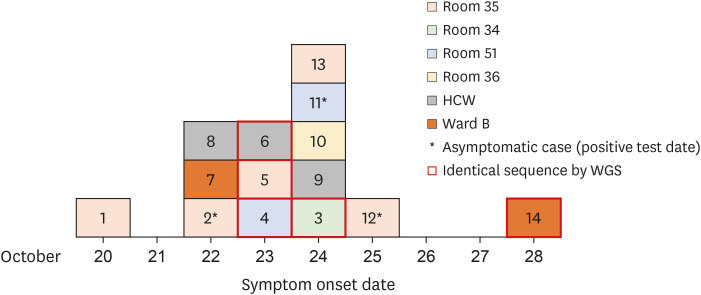
Based on this curve and further epidemiologic investigations, we assumed that case 1 (who had an epidemiologic link with a community outbreak and showed the earliest symptom onset) was the index, and that the remaining cases (2–13) were secondary cases. Of the 12 secondary cases, only four (cases 2, 5, 12, and 13) had close contact with the index. CCTV footage showed that cases 3 and 7 were walking in the corridor at the same time as the index, but for ≤ 15 minutes. In addition, case 14 had not been to ward A; however, she had casual contact with case 7 (being within 2 meters for 3 minutes without conversation). Therefore, this was believed to be a tertiary case who acquired the virus from case 7. Of the 13 secondary and tertiary cases, seven were fully vaccinated and two were partially vaccinated. For the fully vaccinated individuals, the median number of days from the second vaccination to a COVID-19 diagnosis was 142 (interquartile range, 65–145).
Airflow in the resident on-call room
After cases 8 and 9 were confirmed, all resident doctors who stayed in on-call rooms A and B were tested for SARS-CoV-2 PCR every 2 days for 2 weeks. As a result, two out of 12 resident doctors (cases 8 and 9) who stayed in on-call room A tested positive; none of the nine in on-call room B was positive. The air circulator in on-call room A was running continuously (
Supplementary Fig. 1); there was no air circulator in resident on-call room B. Residents in on-call room A stated that they always opened the door. A smoke test showed that when the air circulator was turned on, the air was drawn into the room from the corridor. CCTV footage showed the index patient (case 1) walking and talking in the corridor outside the resident on-call room.
WGS
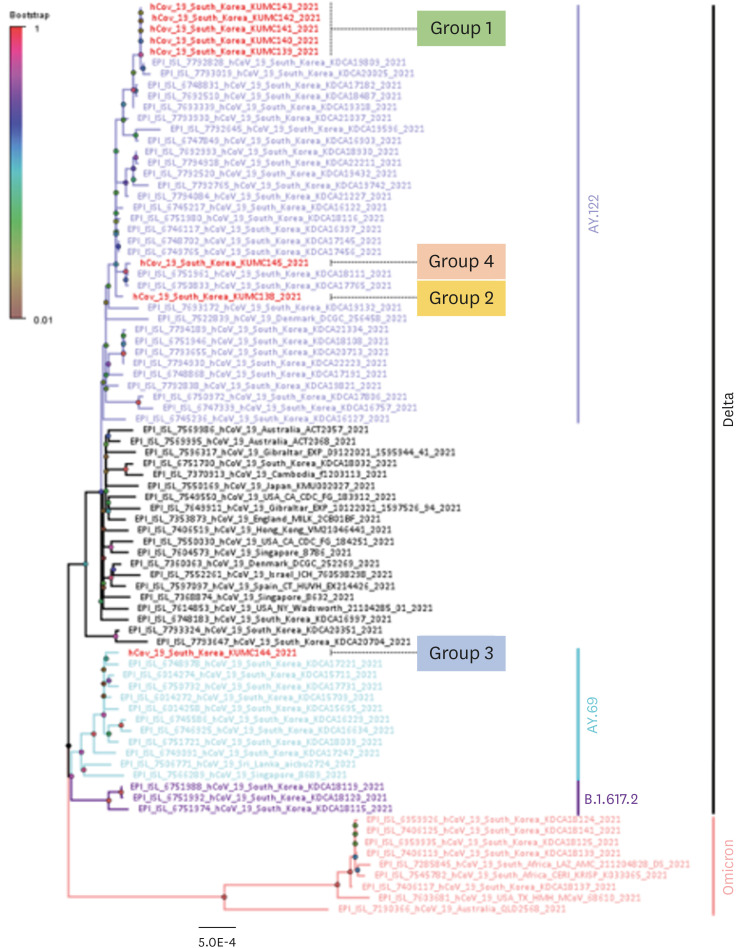
WGS analysis revealed that of the 14 individuals diagnosed with COVID-19, samples from five were available for WGS (cases 3–6, and 14), along with samples from three other non-epidemiologic-linked cases at our hospital. All eight were the delta variant. Four cases in the cluster (4–6, and 14) harbored identical sequences; the sequence from case 3 showed single nucleotide difference, suggesting that all five acquired SARS-CoV-2 from an identical source (group 1). The three samples without an epidemiologic link to the cluster on wards A and B all showed different sequences (groups 2, 3, and 4) (
Fig. 3).
Fig. 3
Phylogenetic tree of severe acute respiratory syndrome coronavirus 2 sequences from Asan Medical Center. A phylogenetic tree was constructed using the maximum likelihood method (1000 replicates) in MEGA X Software. Bootstrap scores are shown at each node. Different branch colors represent different clades and subclades. The scale bar indicates the number of nucleotide substitution per site.
DISCUSSION
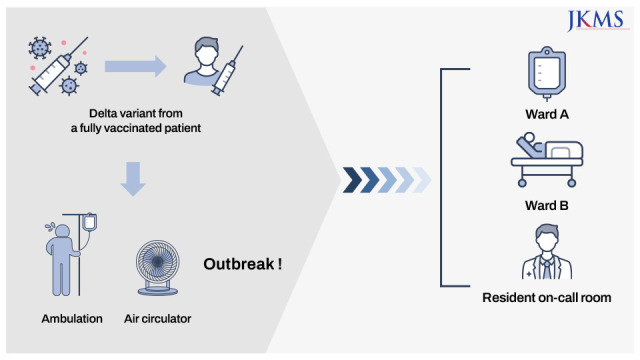
Here, we report a nosocomial cluster of the SARS-CoV-2 delta variant (13 secondary and tertiary cases) arising from a fully vaccinated patient. Transmission appeared to be facilitated by several factors: delayed diagnosis of the index case despite him showing symptoms; frequent ambulation of the index patient in hospital corridors; and use of an air circulator that may have moved contaminated air from the corridor to the on-call room.
Growing evidence shows that vaccinated individuals are less likely to transmit the virus than non-vaccinated individuals.
45 However, there are a few reports of nosocomial outbreaks from a fully vaccinated index. A study from Israel reported a nosocomial outbreak involving 42 patients, staff, and family members of a fully vaccinated patient during the period when the delta variant was dominant.
7 Although the majority of cases were fully vaccinated, the interval from vaccination to diagnosis was a median 25 weeks (≤ 6 months). Therefore, authors suggested that waning immunity was the likely reason for this large outbreak. Similarly, we found that the median number of days from vaccination to diagnosis in fully vaccinated contacts was 142 days (≤ 5 months). Therefore, waning immunity may contribute to transmission. Although vaccination is a critical tool for reducing transmission, other preventive strategies and infection control measures are still needed, as suggested by previous reports of nosocomial outbreaks during the delta era.
89 Testing of the index was performed 2 days after symptom onset; thus diagnosis and isolation were delayed. Previously, we found that the infectors who transmit the virus tended to have a longer duration from symptom onset to isolation than the non-infectors (
P = 0.083).
6 Therefore, the importance of subjective symptom screening of inpatients and caregivers cannot be overemphasized, although this is difficult to undertake in a real-world setting.
We observed frequent transmission to non-close contacts; indeed, less than one third of cases were classified as close contacts. Previously, we reported that more than one quarter of SARS-CoV-2 transmissions in the pre-delta era occurred among non-close contacts.
10 As the delta variant is more transmissible than strains identified before the delta variant, the proportion of non-close contacts among total contacts might increase. Although we could not perform WGS for all cases, we found that at least three of the casual contacts, including patients who stayed in other rooms, a nurse, and one additional causal contact from a secondary case, were cluster-related. On the other hand, common infection in non-close may be the limitation of the epidemiologic investigation through interviews and CCTV footage analysis. Further studies are needed to ascertain the proportion of non-close contacts that is eventually diagnosed with SARS-CoV-2 infection (delta and/or Omicron variants).
Although cases 7 and 8 had no contact with the index or other cases, we believe that they had a spatial association. They stated that they opened the door of the on-call room and always turned on the air circulator. Thus, we performed an airflow study using a smoke test. When the air circulator was turned on, the airflow was directed from the corridor to the on-call room. While two of 12 resident doctors contracted COVID-19 while staying in the on-call room with the air circulator on, none of nine resident doctors who stayed in another on-call room without air circulation became infected. Because the index case walked frequently in the corridor in front of the on-call room, we believe that the air circulator may have facilitated transmission. This finding provides us with important information about the cautious use of re-circulating devices in hospital settings.
This study has several limitations. First, we did not perform WGS for all samples as many specimens were unsuitable due to the low viral load. In particular, specimens from resident doctors staying in the on-call room were unavailable; thus they might not be cluster-related cases. However, thorough epidemiologic investigation and contact tracing revealed no other potential sources in these two cases. Second, we could not perform computational analysis of the airflow study results. Finally, there is a lack of direct evidence ruling out the presence of another potential index patient (the only real evidence for the index case was that he showed the earliest symptom onset). However, extensive epidemiologic interviews by our infection control team identified no other patients who had epidemiologic links with other outbreaks in the community. Case 2 was asymptomatic, with a high Ct value (low viral load), and she had stayed on the ward since October 13, with a negative PCR test on October 16. Therefore, it is less likely that case 2 was the index.
In conclusion, rapid nosocomial transmission of SARS-CoV-2 can occur from a fully vaccinated individual. Universal masking, adequate ventilation, not using equipment that change the airflow, and early recognition of symptoms by rapid testing remain important for prevention of nosocomial outbreaks.






 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download