Abstract
Objective
Recurrent pregnancy loss (RPL) is a fertility problem for which no exact mechanism of abortion or efficient treatment has been described. This study was conducted between 2018 and 2019 to investigate the effectiveness of autologous platelet-rich plasma (PRP) in improving the live birth rate of women with RPL who required in vitro fertilization (IVF).
Methods
A total of 63 patients with at least two previous pregnancy losses and no specific cause detected for the RPL were included and randomly assigned into two groups (PRP and control). Intrauterine infusion of 0.5 mL of autologous PRP was performed 48 hours before embryo transfer in the PRP group. Women in the control group received standard treatment.
Results
Forty patients completed the study. The baseline and cycle characteristics of the participants did not differ significantly between the PRP and control groups. The clinical pregnancy rate was higher in the PRP group (35% vs. 20%, P=0.288). The live birth rate was 15% in the PRP group, but no live births were recorded in the control group (P=0.231).
Recurrent pregnancy loss (RPL) is defined as the spontaneous loss of two or more consecutive pregnancies [1,2]. Approximately 1–3% of reproductive-age couples are estimated to have this fertility problem [1]. Although RPL is associated with many etiologic factors, including genetic, inflammatory, infectious, anatomic, and endocrinologic factors, the cause of RPL in the majority of patients remains unexplained [2]. One of the etiologies underlying RPL is derangement in the endometrial environment [3].
Various elements, such as growth factors, chemokines, and cytokines, play crucial roles in promoting endometrial tissue remodeling during decidualization, yielding the correct environment for the developing conceptus [4,5]. However, alterations in the balance between uteroplacental vascular development and growth factors, such as vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), transforming growth factor (TGF-β), and platelet-derived growth factor (PDGF), may lead to inappropriate angiogenesis, a fundamental requirement for fetal survival and normal progression of pregnancy [6].
Growing evidence suggests that distorted endometrial receptivity may be associated with RPL [7,8]. Decidual transformation of endometrial stromal cells, a critical process for embryo implantation and subsequent fetal growth, may be diminished during decidualization in patients with RPL [7]. The level of TGF-β, one of the elements involved in decidualization events, angiogenesis, and placental function, is reduced in the decidua of patients with RPL [6,9–11]. VEGF is another growth factor that plays a crucial role in both the implantation and initiation of angiogenesis [11], and VEGF polymorphism has been reported to be associated with RPL [11,12].
Platelet-rich plasma (PRP) is an autologous biological product containing platelets in concentrated plasma. PRP is also a source of easy access to chemokines, cytokines, and growth factors [13]. The use of PRP has recently emerged as a therapeutic strategy in various areas of medicine. Platelet granules in PRP contain high concentrations of growth factors, including PDGF, TGF-β, VEGF, FGF, hepatocyte growth factor (HGF), and epidermal growth factor (EGF) [14,15]. In addition, PRP contains serotonin, adenosine, calcium, dopamine, histamine, diphosphate, catecholamines, insulin-like growth factors I and II (IGF I and II), connective tissue growth factor (CTGF), interleukin-1 (IL-1), and interleukin-8 (IL-8) [14–16].
Because of the lack of evidence-based therapeutic recommendations for the treatment of patients with RPL [2,17], optimization of intrauterine circumstances and decidualization may be an efficient therapeutic strategy for these patients [17]. Autologous intrauterine infusion of PRP, as a form of personalized medicine, before embryo transfer in assisted reproductive technology, may be useful for proper implantation in patients with recurrent implantation failure or patients with inadequate endometrial thickness [16,18,19]. Therefore, in this study, we investigated the effectiveness of intrauterine infusion of PRP in improving endometrial derangements in patients with RPL undergoing intracytoplasmic sperm injection (ICSI) treatment.
This prospective, randomized controlled study was conducted at the in vitro fertilization (IVF) center of Taleghani Hospital in Tehran from December 2019 to August 2020. This study was approved by the Ethics Committee of the Shahid Beheshti University of Medical Sciences (IR.SBMU.REC.1398.079, approval date: November 2019) and registered at https://en.irct.ir/trial/41765. Informed consent was obtained from all participants before entering the study.
We included women with a history of two or more pregnancy losses before 20 weeks of gestation who were candidates for ICSI, aged below 40 years, and had body mass index (BMI) of 20–30 kg/m2. The exclusion criteria were immunological or hematological disorders, antiphospholipid antibody syndrome, endometriosis, testicular sperm extraction, oocyte donation, hormonal or chromosomal abnormalities, and anatomical uterine disorders. The sample size was determined using the following formula:
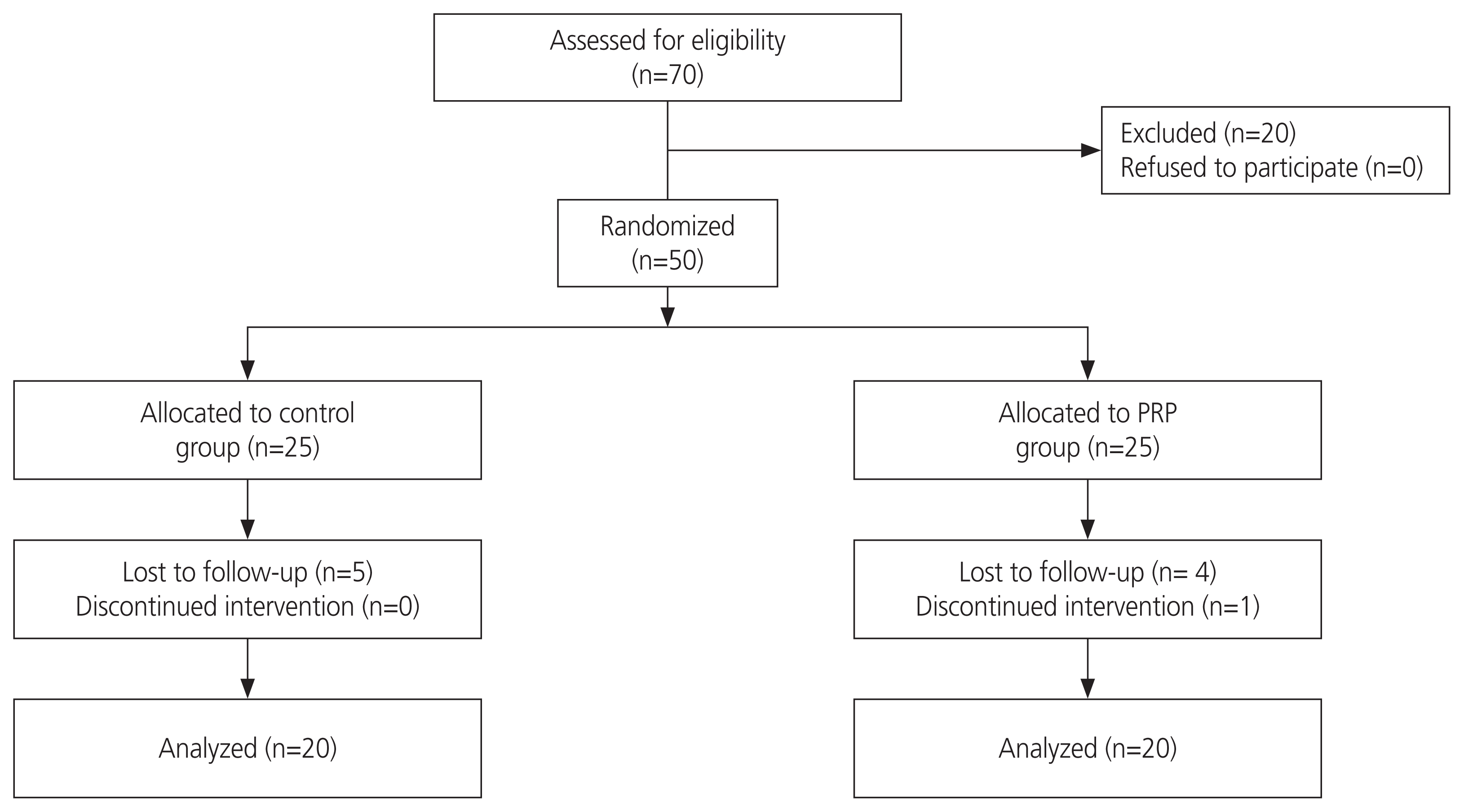
When α=0.05, Zα=1.96, β=0.20, Zβ=0.84, and effect size=0.8. The sample size was 25 patients in each group. Among the 50 patients included in the current study, 10 were unable to complete the study procedure (Fig. 1). The remaining 40 patients were randomly categorized into two groups (PRP and control groups) using computer-generated simple random tables in a 1:1 ratio.
All participants underwent ovarian stimulation with the standard gonadotropin-releasing hormone (GnRH) antagonist protocol. Ovarian stimulation was initiated with exogenous gonadotropin (225–450 IU daily; Pergoveris, Merck-Serono, Roma, Italy) on the third day of the menstrual cycle. Follicular growth was monitored after 6 days by ultrasonography, and when follicles with a diameter of 14 mm were visualized, GnRH antagonist treatment was initiated with Cetrorelix acetate (0.25 mg daily; Cetrotide, Serono, London, UK). When at least three follicles with diameter >17 mm were visualized by ultrasound, ovulation was triggered using human chorionic gonadotropin (Pregnyl, MSD, Brussels, Belgium). Thirty-six hours after the ovulation trigger, oocyte retrieval was performed, and metaphase II oocytes were inseminated by ICSI.
In the PRP group, intrauterine administration of PRP was performed under ultrasound guidance 48 hours before embryo transfer. PRP was obtained from 8.5 mL of autologous peripheral venous blood by using a two-step process. The blood sample was collected into a syringe containing 1.5 mL of acid citrate A anticoagulant solution (ACD-A, Arya Mabna Tashkhis, Tehran, Iran) and immediately centrifuged at 1,200 rpm for 10 minutes to separate the red blood cells. The solution was centrifuged again at 3,300 rpm for 5 minutes to obtain PRP-containing platelets approximately 4–5 times more than circulating blood. Approximately 0.5 mL of PRP was infused into the uterine cavity using a catheter (Takvin Teb Co., Tehran, Iran) before embryo transfer.
Five days after ICSI, when endometrial thickness reached ≥7 mm, one or two fresh blastocyst embryos were transferred to the uterine cavity. The luteal phase was supported with vaginal progesterone (400 mg twice daily; Cyclogest, Hoechst, Hounslow, UK). Clinical pregnancy was defined by the presence of an embryonic sac at 5–6 weeks gestation. Spontaneous abortion was defined by the loss of pregnancy before 20 weeks of gestation, and live birth was defined as birth after 24 weeks of gestation [20].
For statistical analysis, an independent t-test, Mann-Whitney test, Fisher’s exact test, and chi-squared test were performed. Continuous variables were presented as mean±standard deviation or median and interquartile range, and categorical variables were presented as frequency (percentage). The primary outcome measures were clinical pregnancy, abortion, ectopic pregnancy, and live birth rates. Logistic regression analysis was performed to adjust the outcome measures of the study for confounding variables. Statistical analysis was performed using SPSS version 21 (IBM Corp., Armonk, NY, USA). P-values <0.05 were considered statistically significant.
Data from 40 patients who underwent IVF cycles were analyzed in this study: 20 patients in the control group with standard treatment and 20 patients in the PRP group. The baseline characteristics of the participants (female age, BMI, serum anti-Müllerian hormone [AMH] level, basal follicle-stimulating hormone level, infertility duration, primary or secondary infertility, number of previous abortions, number of previous IVF cycles, and etiology of infertility) did not significantly differ between the two groups (Table 1). The cycle parameters and treatment outcomes of the study groups are presented in Table 2. No significant difference was observed in the number of total oocytes retrieved, number of metaphase II oocytes, the ratio of metaphase II oocytes/total oocytes, fertilization rate, number of embryos transferred, abortion rate, and ectopic pregnancy (Table 2). The clinical pregnancy rate was higher in the PRP group (35% vs. 20%). The live birth rate was 15% in the PRP group, but no live births were recorded in the control group (Table 2). The results of multivariable analyses to adjust the main outcome measures of the study for confounding variables (infertility duration, number of previous IVF cycles, and AMH) are shown in Table 3.
Good endometrial receptivity and decidualization are the determinants of proper implantation, subsequent embryonic development, and successful gestation. Thus, a disrupted endometrial state in the early stages of pregnancy can have detrimental effects on the continuation of the pregnancy. However, the exact mechanism underlying abortion in RPL patients is not yet clear, and no efficient treatment is available for infertile couples with RPL [21]. According to the available evidence, optimization of the endometrial status during implantation may be the key to proper decidualization and angiogenesis and prevention of abortion in patients with RPL [7,8,17]. The current study is the first investigation, to the best of our knowledge, evaluating the effectiveness of PRP infusion into the uterus of RPL women in improving the live birth outcome after IVF.
In our previous clinical trials, PRP infusion into the uterus improved endometrial expansion in patients with a refractory thin endometrium [22] and pregnancy outcomes in patients with recurrent implantation failure [16]. In this study, we showed that infusion of PRP into the uterus before embryo transfer in patients with RPL could increase the rate of live births, although this effect was not statistically significant. No live births were recorded in the control group in this study, but the live birth rate in the patients treated with PRP was 15% without any complications. Notably, the observed statistically insignificant increase in the live birth rate among PRP-treated patients may also be attributable to the limitations of the population studied. Since the exact mechanism of abortion in women with RPL is unknown and this is the first investigation, to our knowledge, regarding the treatment of RPL patients with PRP, we conducted this pilot study with a limited number of patients. Accordingly, our results suggest that infusion of PRP into the uterus may be a safe and effective treatment for women with RPL.
In recent years, PRP has been widely suggested to improve endometrial quality in the treatment of various infertility issues, such as thin endometrium [23–25], Asherman syndrome [26], recurrent implantation failure [16,24], and severe intrauterine adhesion [27]. PRP is an inexpensive and easy to obtain autologous resource. The factors in PRP include PDGF, TGF-β, VEGF, FGF, HGF, EGF, serotonin, adenosine, calcium, dopamine, histamine, diphosphate, catecholamines, IGF I and II, CTGF, IL-1, and IL-8 [14–16]. The chemokines, cytokines, and growth factors contained in PRP may improve endometrial conditions and lead to proper vascularization, decidualization, and continued gestation.
Appropriate angiogenesis is crucial for the formation of the decidual capillary network, embryonic growth, and continuation of pregnancy. Thus, inadequate vascular development and growth, which leads to hypoxia and insufficient blood flow, may be involved in RPL [8]. Multiple angiogenic factors play essential roles in vascularization of the chorionic villi and decidua. Factors such as TGF-β, PDGF, and VEGF have been reported to be dysregulated in the endometrium of women with a history of RPL [10–12,28]. Alterations in the ratio of the concentrations of growth factors in the uterus can cause dysregulation of decidualization and implantation.
PRP maintains the normal physiological ratios of growth factors, chemokines, and cytokines in the endometrium. The elements in PRP, including VEGF, IGF, FGF, TGF-β, and IL-1, play fundamental roles in angiogenesis and decidualization [6,9,29]. Therefore, by improving decidualization and angiogenesis, PRP infusion into the uterus may inhibit embryonic growth failure and enhance the chance of live birth in patients with RPL.
This pilot study demonstrated that infusion of PRP into the uterus of patients with RPL before embryo transfer in the ICSI cycle could increase the chance of live birth, although this effect was not statistically significant. We suggest that PRP, as a safe, autologous, low-cost, and effective therapeutic agent, may improve endometrial circumstances in the early stages of gestation and lead to successful live births among RPL patients. Further randomized controlled trials with adequate study populations are required.
Notes
Ethical approval
This study was approved by the Ethics Committee of the Shahid Beheshti University of Medical Sciences (IR.SBMU. REC.1398.079, approval date: November 2019) and registered at https://en.irct.ir/trial/41765.
References
1. Egerup P, Kolte AM, Larsen EC, Krog M, Nielsen HS, Christiansen OB. Recurrent pregnancy loss: what is the impact of consecutive versus non-consecutive losses? Hum Reprod. 2016; 31:2428–34.

2. The ESHRE Guideline Group on RPL, Bender Atik R, Christiansen OB, Elson J, Kolte AM, Lewis S, et al. ESHRE guideline: recurrent pregnancy loss. 1st ed. Oxford: Oxford University Press;2018.
3. Ticconi C, Pietropolli A, Di Simone N, Piccione E, Fazleabas A. Endometrial immune dysfunction in recurrent pregnancy loss. Int J Mol Sci. 2019; 20:5332.

4. Di Nicuolo F, D’Ippolito S, Castellani R, Rossi ED, Masciullo V, Specchia M, et al. Effect of alpha-lipoic acid and myoinositol on endometrial inflammasome from recurrent pregnancy loss women. Am J Reprod Immunol. 2019; 82:e13153.

5. Baradwan S, Abdulghani SH, Abuzaid M, Khadawardi K, Alshahrani MS, Al-Matary A, et al. 17-alpha hydroxy-progesterone caproate for the prevention of recurrent preterm birth among singleton pregnant women with a prior history of preterm birth: a systematic review and meta-analysis of six randomized controlled trials. Obstet Gynecol Sci. 2021; 64:484–95.

6. Bagheri A, Chianeh YR, Rao P. Role of angiogenic factors in recurrent pregnancy loss. Int J Reprod Contracept Obstet Gynecol. 2013; 2:497–503.

7. Dhaenens L, Lierman S, De Clerck L, Govaert E, Deforce D, Tilleman K, et al. Endometrial stromal cell proteome mapping in repeated implantation failure and recurrent pregnancy loss cases and fertile women. Reprod Biomed Online. 2019; 38:442–54.

8. Tan SY, Hang F, Purvarshi G, Li MQ, Meng DH, Huang LL. Decreased endometrial vascularity and receptivity in unexplained recurrent miscarriage patients during midluteal and early pregnancy phases. Taiwan J Obstet Gynecol. 2015; 54:522–6.

9. Daher S, de Arruda Geraldes Denardi K, Blotta MH, Mamoni RL, Reck AP, Camano L, et al. Cytokines in recurrent pregnancy loss. J Reprod Immunol. 2004; 62:151–7.

10. Lea RG, Underwood J, Flanders KC, Hirte H, Banwatt D, Finotto S, et al. A subset of patients with recurrent spontaneous abortion is deficient in transforming growth factor beta-2-producing “suppressor cells” in uterine tissue near the placental attachment site. Am J Reprod Immunol. 1995; 34:52–64.

11. An HJ, Kim JH, Ahn EH, Kim YR, Kim JO, Park HS, et al. 3′-UTR polymorphisms in the vascular endothelial growth factor gene (VEGF) contribute to susceptibility to recurrent pregnancy loss (RPL). Int J Mol Sci. 2019; 20:3319.

12. Sun Y, Chen M, Mao B, Cheng X, Zhang X, Xu C. Association between vascular endothelial growth factor polymorphism and recurrent pregnancy loss: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2017; 211:169–76.

13. Gobbi G, Vitale M. Platelet rich plasma for biological therapy: applications and limits. Maffulli N, editor. Platelet rich plasma in musculoskeletal practice. London: Springer;2016. p. 175–98.

14. Mejia HA, Bradley JP. The effects of platelet-rich plasma on muscle: basic science and clinical application. Oper Tech Sports Med. 2011; 19:149–53.

15. Wasterlain AS, Braun HJ, Dragoo JL. Contents and formulations of platelet rich plasma. Maffulli N, editor. Platelet rich plasma in musculoskeletal practice. London: Springer;2016. p. 1–29.

16. Nazari L, Salehpour S, Hosseini MS, Hashemi Moghanjoughi P. The effects of autologous platelet-rich plasma in repeated implantation failure: a randomized controlled trial. Hum Fertil (Camb). 2019; 23:209–13.

17. Kuroda K. Impaired endometrial function and unexplained recurrent pregnancy loss. Hyperten Res Pregnancy. 2019; 7:16–21.

18. Sipahi M. Effects of autologous platelet-rich plasma on endometrium thickness and pregnancy rates during intrauterine insemination. Middle Black Sea J Health Sci. 2019; 5:63–6.

19. Nazari L, Salehpour S, Hoseini S, Zadehmodarres S, Ajori L. Effects of autologous platelet-rich plasma on implantation and pregnancy in repeated implantation failure: a pilot study. Int J Reprod Biomed. 2016; 14:625–8.
20. Coksuer H, Akdemir Y, Ulas Barut M. Improved in vitro fertilization success and pregnancy outcome with autologous platelet-rich plasma treatment in unexplained infertility patients that had repeated implantation failure history. Gynecol Endocrinol. 2019; 35:815–8.
21. Jaslow CR, Carney JL, Kutteh WH. Diagnostic factors identified in 1020 women with two versus three or more recurrent pregnancy losses. Fertil Steril. 2010; 93:1234–43.

22. Nazari L, Salehpour S, Hoseini S, Zadehmodarres S, Azargashb E. Effects of autologous platelet-rich plasma on endometrial expansion in patients undergoing frozen-thawed embryo transfer: a double-blind RCT. Int J Reprod Biomed. 2019; 17:443–8.

23. Kim H, Shin JE, Koo HS, Kwon H, Choi DH, Kim JH. Effect of autologous platelet-rich plasma treatment on refractory thin endometrium during the frozen embryo transfer cycle: a pilot study. Front Endocrinol (Lausanne). 2019; 10:61.

24. El Hamedi MA, Salem HA. Platelet rich plasma in repeated implantation failure in women with thin endometrium thickness. Egypt J Hosp Med. 2019; 77:5873–5.

25. Zadehmodarres S, Salehpour S, Saharkhiz N, Nazari L. Treatment of thin endometrium with autologous platelet-rich plasma: a pilot study. JBRA Assist Reprod. 2017; 21:54–6.

26. Molina A, Sánchez J, Sánchez W, Vielma V. Platelet-rich plasma as an adjuvant in the endometrial preparation of patients with refractory endometrium. JBRA Assist Reprod. 2018; 22:42–8.
27. Torky AMMAE, Amer MIM, Ahmed MES, Kamal RM. The value of using platelet rich plasma after hysteroscopic analysis of severe intrauterine adhesions (a randomized controlled trial). Egypt J Hosp Med. 2018; 71:2869–74.
Table 1
Baseline characteristics of the patients
Table 2
Characteristics of intracytoplasmic sperm injection cycles
| Parameter | Control (n=20) | PRP (n=20) | P-value |
|---|---|---|---|
| Total oocytes retrieved | 8 (4–11) | 9 (6–12) | 0.625 |
| Metaphase II oocytes | 5 (2–8) | 7 (3–9) | 0.327 |
| Ratio of metaphase II oocytes/total oocytes | 0.85 (0.58–0.97) | 0.89 (0.68–0.96) | 0.495 |
| Fertilization rate (%) | 76.38 (45.0–100.0) | 73.21 (52.77–91.98) | 0.817 |
| Embryos transferred | 1.000 | ||
| 1 | 3 (15) | 3 (15) | |
| 2 | 17 (85) | 17 (85) | |
| Clinical pregnancy | 4 (20) | 7 (35) | 0.288 |
| Abortion | 4 (20) | 4 (20) | 1.000 |
| Ectopic pregnancy | 1 (5) | 0 (0) | 1.000 |
| Live birth | 0 (0) | 3 (15) | 0.231a) |
Table 3
Multivariable analysis of potential factors associated with outcome measures
| Parameter | Crude OR | P-value | Adjusteda) OR | P-value |
|---|---|---|---|---|
| Pregnancy | 2.15 (0.5–9.0) | 0.293 | 2.15 (0.4–11.58) | 0.370 |
| Abortion | 1.00 (0.21–4.70) | 1.000 | 1.15 (0.91–1.45) | 0.243 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download