I. Introduction
The outlook of artificial intelligence for healthcare (AI4H) is very promising [
1]. The size of the AI4H market with software as a medical device (SaMD) is expected to reach about US $4.9 billion in 2020 and $45.2 billion in 2026, with an annual growth rate of 44.9%. In particular, growth from US $1.9 billion to $17.8 billion (an annual growth rate of 45.6%), $1.4 billion to $12.3 billion (44.5%), and $1.2 billion to $12.0 billion (46.3%) in the United States, Europe, and Asia-Pacific in this period [
2,
3]. AI4H is used for many healthcare applications, such as clinical decision support, virtual assistants, computer-aided diagnosis, and robots in hospitals [
4]. As a result, the number of Food and Drug Administration (FDA)-approved AI-based algorithms in the United States has increased from 41 to 68, and in South Korea from 4 to 61, between January 2018 and December 2020 [
5,
6]. As described previously [
7], there are 130 US FDA-approved medical AI devices.
Three fundamental factors have contributed to the recent achievements of AI4H: (1) advances in AI algorithms, (2) improvements in computing power such as general-purpose computing on graphics processing units, and (3) generation of healthcare big data such as Electronic Medical Records, genomic sequencing data, and computed tomography and magnetic resonance imaging (CT/MRI) scans. These factors converge to enable research on AI-based prognosis prediction, disease diagnosis, genomics, and radiomics. Some researchers have reported that AI outperformed physicians in disease classification, demonstrating its success in detecting diseases on medical images [
7–
9].
To address the success and trends of AI4H, several white papers and reports have been published, such as the AI index report from Stanford University [
10] and the AI in healthcare report from the National Academy of Medicine [
11]. Recently, a survey article identifying the expected impact and value of AI4H was published, based on an online survey with 151 experts from 15 countries in Europe. They divided challenges into technical and non-technical, for example data anonymization, sharing and regulatory issues, and standardization [
12]. However, most of those reports have focused on showing current issues without proposing a practical discussion for real-world use. There have been a few previous studies discussing the practicality and challenges of AI4H in Korea [
13,
14], but no study has yet discussed the issues of AI4H from challenge identification to solutions in terms of stakeholders’ opinions, which will be very critical for practical applications.
The aim of this study is identifying stakeholders’ requirements for AI4H in Korea by reviewing governmental research and development funding and conducting an online survey and in-depth expert interviews. This study will help to promote future research and business of AI4H in Korea.
III. Results
1. Analysis of Governmental Research Funding
First, a total of 299 research projects were found in the NTIS database according to the inclusion criteria. After manually reviewing the candidate projects, 115 duplicated multi-year projects and 24 irrelevant projects were excluded. Finally, 160 projects were selected and analyzed.
The major data types used for AI4H during the past 5 years are shown in
Table 1. As expected, among the 160 projects, the most common data type was radiology images (95 projects, 59.4%). Others (29 projects, 18.1%), including patient-reported outcome or digital phenotypes, ranked second. Laboratory data (6.9%), radiology/pathology reports (5.6%), and genomic data (5.0%) followed. The number of projects increased sharply in 2017.
Table 1
Major data types during the past 5 years
|
2015 |
2016 |
2017 |
2018 |
2019 |
All |
|
CT/MRI |
2 (66.7) |
2 (40.0) |
22 (57.9) |
37 (62.7) |
32 (58.2) |
95 (59.4) |
|
Lab data |
0 (0) |
0 (0) |
4 (10.5) |
4 (6.8) |
3 (5.5) |
11 (6.9) |
|
Genome |
0 (0) |
1 (20.0) |
1 (2.6) |
2 (3.4) |
4 (7.3) |
8 (5.0) |
|
Vital |
0 (0) |
0 (0) |
2 (5.3) |
2 (3.4) |
3 (5.5) |
7 (4.4) |
|
Radiology/pathology reports |
1 (33.3) |
0 (0) |
2 (5.3) |
2 (3.4) |
4 (7.3) |
9 (5.6) |
|
Free text reports |
0 (0) |
1 (20.0) |
0 (0) |
0 (0) |
0 (0) |
1 (0.6) |
|
Othersa
|
0 (0) |
1 (20.0) |
7 (18.4) |
12 (20.3) |
9 (16.4) |
29 (18.1) |
|
All |
3 (100) |
5 (100) |
38 (100) |
59 (100) |
55 (100) |
160 (100) |

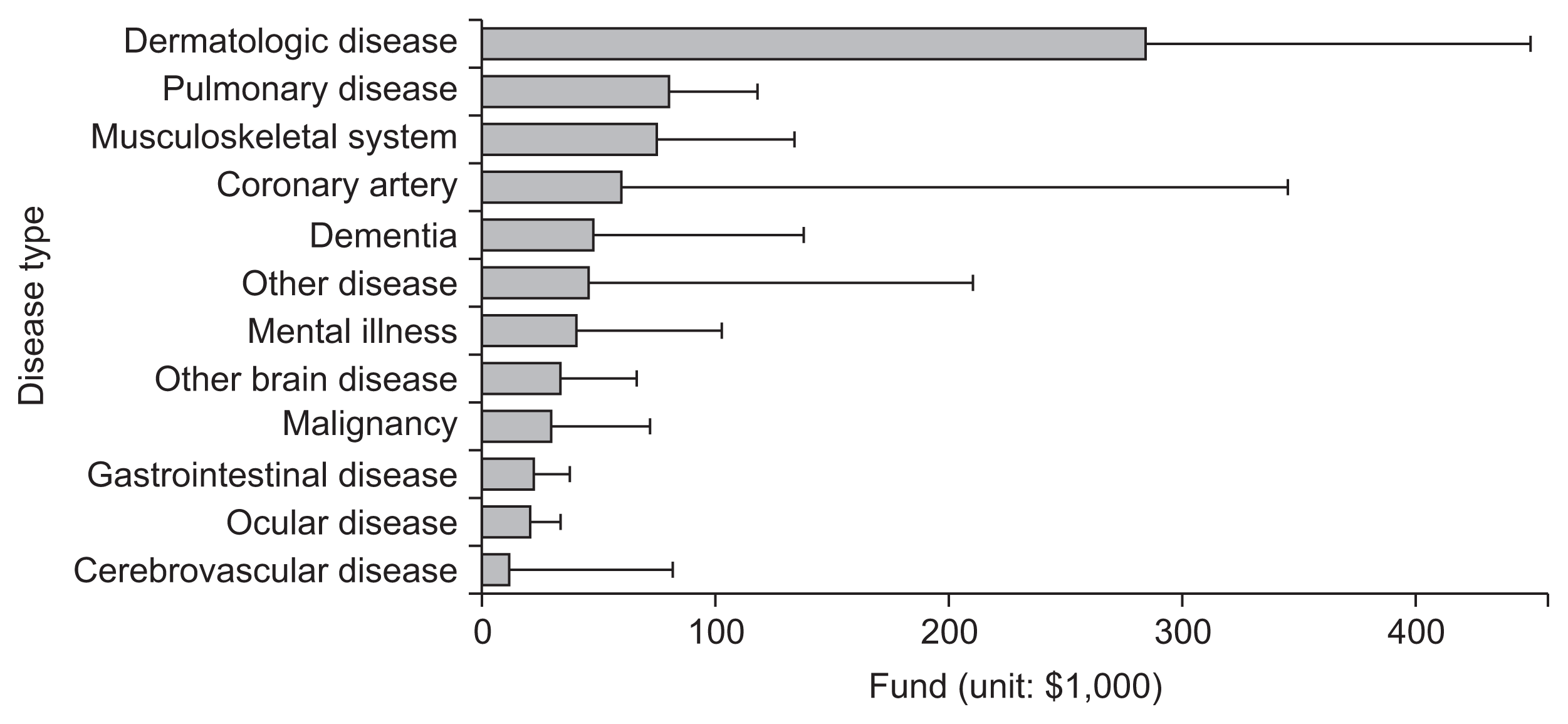
Figure 1 depicts the average funding by target disease type per year. The unit of the funding scale is US $1,000. Dermatology-related diseases received the most funds (US $284,000), followed by pulmonary diseases (US $80,000), musculoskel et al system disorders (US $75,000), coronary artery diseases (US $60,000) and dementia (US $48,000).
Figure 1
Average funding by target disease per year.

2. Survey
A total of 101 KOSAIM members participated in the survey, 54 (53.5%) of whom worked in hospitals, 28 (27.7%) in academia, and 19 (18.8%) in industry.
Among the data demands in hospitals and academia, most of the responses indicated image types (27.6% and 20.5%) and laboratory data (22.1% and 16.7%). In contrast, researchers in industry expressed the highest demand for laboratory data (20.8%), followed by free-text reports (18.8%) and radiology/pathology reports (18.8%). The demand for free-text report analysis was higher than that for vital signs in hospitals and industry, but lower in academia (
Table 2).
Table 2
Data demands for AI development
|
Hospital |
Industry |
Academia |
All |
|
CT/MRI |
45 (27.6) |
8 (16.7) |
16 (20.5) |
69 (23.9) |
|
Lab data |
36 (22.1) |
10 (20.8) |
13 (16.7) |
59 (20.4) |
|
Genome |
10 (6.1) |
4 (8.3) |
12 (15.4) |
26 (9.0) |
|
Vital |
22 (13.5) |
7 (14.6) |
13 (16.7) |
42 (14.5) |
|
Radiology/pathology reports |
23 (14.1) |
9 (18.8) |
10 (12.8) |
42 (14.5) |
|
Free text reports |
25 (15.3) |
9 (18.8) |
10 (12.8) |
44 (15.2) |
|
Othersa
|
2 (1.2) |
1 (2.1) |
4 (5.1) |
7 (2.4) |
|
All |
163 (100) |
48 (100) |
78 (100) |
289 (100) |

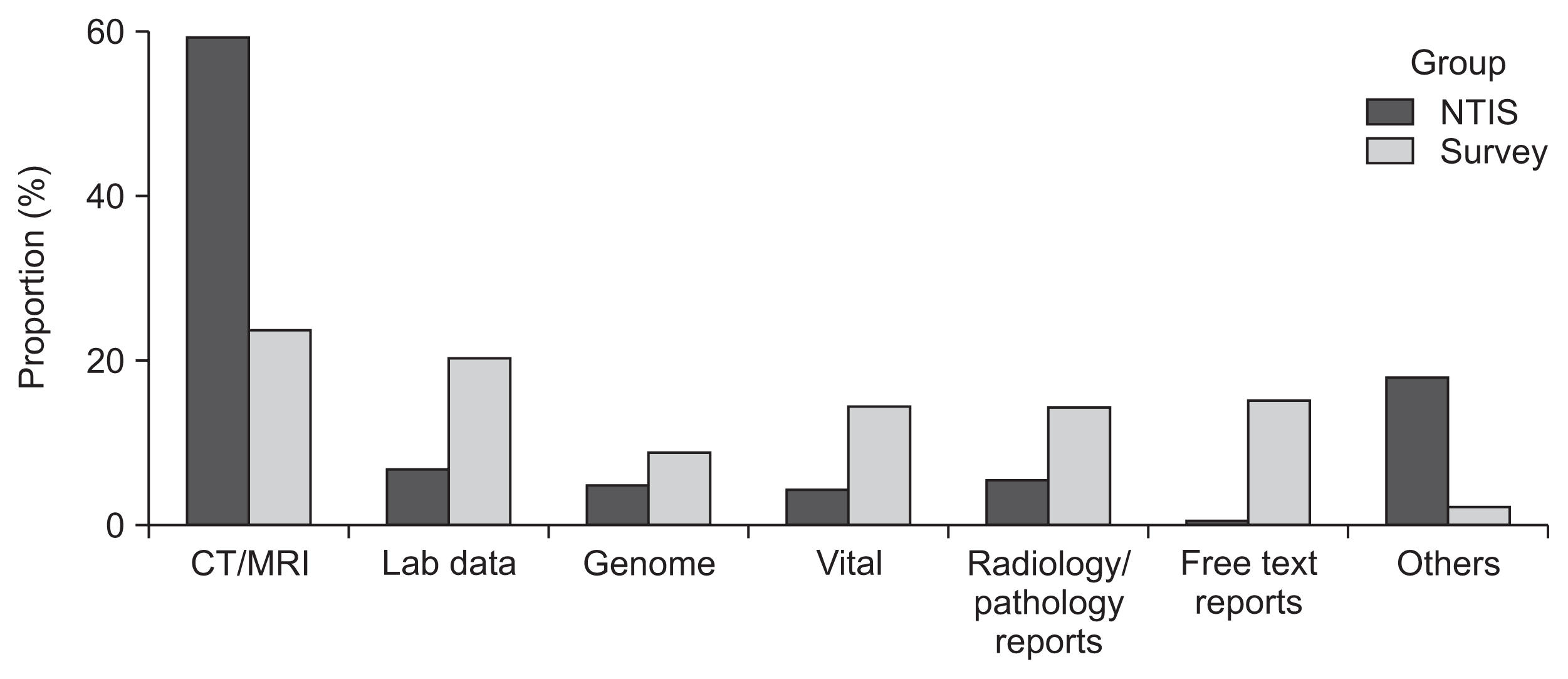
Figure 2 shows the results of a comparison of demand for data types between NTIS and survey responses. The patterns of data demands were significantly different (
p < 0.05), especially regarding medical image data (59.4% for NTIS and 23.9% for survey responses).
Figure 2
Comparison of data types between NTIS (from 2015 to 2019) and the KOSAIM members’ survey in 2020.

Over half of AI4H software was related to the diagnosis prediction (33.3%) or prognosis prediction (31%). Interestingly, researchers in the hospital field, who comprised one-fifth of the respondents, chose treatment plan recommendations (19.4%) (
Table 3).
Table 3
Solution demands for AI development
|
Hospital |
Industry |
Academia |
All |
|
Diagnosis prediction |
35 (32.4) |
8 (32.0) |
14 (36.8) |
57 (33.3) |
|
Prognosis prediction |
32 (29.6) |
8 (32.0) |
13 (34.2) |
53 (31.0) |
|
Monitoring |
16 (14.8) |
5 (20.0) |
6 (15.8) |
27 (15.8) |
|
Treatment plan recommendation |
21 (19.4) |
4 (16.0) |
4 (10.5) |
29 (17.0) |
|
Othersa
|
4 (3.7) |
0 (0) |
1 (2.6) |
5 (2.9) |
|
All |
108 (100) |
25 (100) |
38 (100) |
171 (100) |

3. Expert Interviews
Thirteen experts in the field of medical AI participated in the expert interviews, of whom three (23.1%) worked in hospitals, four (30.8%) in academia, and six (46.1%) in industry.
We identified the major topics, organizing them into five items by interviewer affiliation, as shown in
Table 4.
Table 4
Comparison of major topics by affiliation in the expert interviews
|
Hospital |
Industry |
Academia |
All |
|
Partnership |
2 (5.0) |
5 (10.6) |
0 (0) |
7 (6.3) |
|
Education |
3 (7.5) |
6 (12.8) |
7 (28.0) |
16 (14.3) |
|
Analysis technology |
9 (22.5) |
9 (19.1) |
4 (16.0) |
22 (19.6) |
|
Data |
20 (50.0) |
11 (23.4) |
9 (36.0) |
40 (35.7) |
|
Regulation |
6 (15.0) |
16 (34.0) |
5 (20.0) |
27 (24.1) |
|
All |
40 (100) |
47 (100) |
25 (100) |
112 (100) |

Half of the major topics for AI4H related to data among experts in hospitals. In industry, regulation was the most commonly identified problem (34.0%), followed by data (23.4%). In academia, data were often identified as a problem (36.0%), but respondents also frequently mentioned education (28.0%) and regulation (20.0%). Other topics included medical charges, marketability, practical applications, medicolegal issues, and organization of governance for AI4H.
Table 5 shows the specific keywords. In the hospital field, most experts mentioned needs related to data, such as standardization, a common data model, and data certification. They specifically mentioned that available domestic open datasets are insufficient for technology development and performance evaluation. Moreover, they pointed out that common data models and data standards such as Health Level Seven (HL7) are required. Furthermore, the necessity of an internal organization in charge of hospital data governance was proposed.
Table 5
Keywords of expert interviews
|
Hospital |
Industry |
Academia |
|
Data |
Efficiency evaluation |
Data security |
|
Data certification |
AI clinical application |
Deregulation |
|
Clinical decision support system |
Deregulation |
Anonymity |
|
Data standardization |
Data open |
Pseudonymization |
|
Common data model |
Manpower |
|
|
Vitalization the private-led market |
|
|
Minimize government intervention |
|
|
Partnership with foreign institutions |
|

In the industrial field, experts mainly mentioned regulations, practical efficacy evaluation, and data accessibility. Although the Korean Privacy Information Protection Act has been amended, it is still difficult to use clinical data in practice. Because of this limitation, it is difficult to evaluate medical AI solutions. There is a need for proactive deregulation. The participants also proposed changing the insurance reimbursement plan to promote the adoption of AI4H.
Experts in academia argued for privacy protection and long-term funds. They mentioned the necessity of long-term collaboration between academia, hospitals, and industry. Moreover, some of them discussed the practical applications and responsibilities of AI4H.
Most interviewees, regardless of their position, emphasized the importance of training for AI4H experts. AI4H is a multidisciplinary field requiring knowledge of medical areas and AI technology. Specifically, academically oriented needs of education require more options in methodology of fundamental technology. In contrast, the market needs practical courses for employees who want to develop essential AI4H techniques.
IV. Discussion
According to the increasing demand and interest in AI4H, we aimed to identify the practical needs of AI4H stakeholders. Previous studies have examined the status of AI4H, and our study expanded the research further to address practical issues regarding the AI4H development. We identified demands and trends through an analysis of NTIS data, survey responses, and expert interviews.
The analysis of 5 years of NTIS data showed that many studies focused on disease diagnosis using radiology image data, as shown in
Table 1. AI solutions using image data already have high accuracy and have been commercialized [
5–
6]. Research using radiology image data has tended to decrease from 2018, which could indicate that the research on radiology images has already reached a certain level. As the level of research and technology advances, research on genomic data, psychology, mental illness, and digital phenotypes, which are areas where demand was relatively low, is also increasing. This trend can be seen in the results of the survey; the data demands are diverse, as shown in
Figure 2.
As presented in
Table 2, the data demands of hospitals and academia have similar patterns, but those of industry are different in terms of a focus on non-image data. European researchers also reported the strongest demand for electronic health records and patient-generated data [
12]. Through these results, it can be expected that the research demand and diversity of AI4H will increase further in the future, as exemplified by multi-modal research using radiology images with various data including laboratory results and free text.
The identified issues and requirements are presented in
Table 6. In AI4H, medical data are an essential resource for the development and evaluation of AI models. Since developing new drugs and diagnostic technologies through healthcare big data will eventually lead to improvements in public health, research in this field should be accessible. To address this need, the government must clarify the related laws and regulations. It is also important to strengthen privacy protection using various recent technologies, such as federated learning, homomorphic encryption, synthetic data, differential privacy, and dynamic consent [
17–
21]. Other countries also have been arguing for regulations including data protection and data sovereignty which are very similar considerations [
12].
Table 6
Identified issues and requirements of stakeholders
|
Issue |
Requirement |
|
Laws & regulations |
Clarify the related laws and regulations
Strengthen the privacy protection using the advanced technologies |
|
Utilizing data |
Build the FAIR healthcare big data at national level
Establish data governance organization |
|
Reimbursement |
Reimburse AI SaMDs |
|
Human resource development |
Need long-term educations on AI4H |

We identified needs for cohort and open dataset development supported by the government and the expansion of data quality certification for hospitals. To promote access to patient data in hospitals, it is necessary to establish a national healthcare big data system, such as the “All of Us” project in the United States [
22]. The data should be FAIR (findable, accessible, interoperable, and reusable) [
23]. With the FAIR guideline, data quality should also be guaranteed. Efforts for data quality management through standardization are also needed. To accelerate data quality management, a data certification program might be considered. The Korea Data Agency already runs a data certification center that screens and certifies data quality, data management, and data security to ensure the quality of the information systems used at institutions or companies [
24]. Furthermore, a governance organization is required to manage and supervise the data evaluation methods and standardization of medical data [
25].
Reimbursement for AI SaMD is the biggest issue in Korea, where there were 61 approved AI SaMDs in 2020, but none of them were listed in the National Health Insurance register [
26]. Without reimbursement, it is difficult for hospitals in Korea to use these AI SaMDs. Therefore, a forward-looking policy is necessary. The Digital Health Applications of Germany [
27], US Centers for Medicare & Medicaid Service New Technology Add-on Payment [
28], and US Medicare Coverage of Innovative Technology [
29] could be considered as benchmarks for insurance reimbursement. In terms of real-world applications and regulations, it is also necessary to develop data de-identification technology and ethical guidelines on AI4H.
Well-known institutions offer various AI courses. However, most courses are conducted in the short term and have limited options regarding methodologies of fundamental technology. Furthermore, there are few courses designed for the medical field with accompanying medical domain education. In order to lead and sustain AI4H in the future, long-term human resources development programs should be implemented.
There are several limitations of this study. First, there is the issue of selection bias with participants who presented opinions supporting AI4H. However, we selected KOSAIM members, who are experts in the field of medical AI, in order to obtain reliable results. In addition, the sample sizes of the survey and the expert interviews were relatively small. Second, as we examined research funding only in the NTIS database using the keyword “healthcare AI,” some research projects might have been excluded. Finally, meaningful opinions might have been excluded because the frequency of the terms in the interviews was used for analysis; however, the interview content was sufficiently discussed.
In conclusion, we identified technology, regulatory, and data issues for practical AI4H applications from the perspectives of experts in the hospital, industry, and academia fields in Korea. We found four main issues—laws and regulations, data utilization, reimbursement, and human resource development—and identified requirements for further research in AI4H.




 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download