This article has been
cited by other articles in ScienceCentral.
Abstract
Immune checkpoint inhibitors (ICIs) have emerged as one of the most promising therapeutic options for advanced cancers. While ICIs have improved survival in multiple cancers, their increased use is restricted by various immune-related adverse events. In this report we describe a patient with renal cell carcinoma who received a combination of ICIs, nivolumab plus ipilimumab, and who developed lumbosacral polyradiculoneuropathy. Corticosteroid use was an effective treatment for this patient.
Go to :

Keywords: Polyradiculoneuropathy, Drug-related side effects, Immune checkpoint inhibitors
Immune checkpoint inhibitors (ICIs) are a type of cancer immunotherapy drugs that augment immunologic reactions against cancer cells. ICIs suppress inhibitory signaling pathways that cancer cells use to escape the immune system of the host, known as immune checkpoints.
1 Immunotherapy using ICIs has revolutionized cancer treatment paradigms and improved survival in various advanced cancer patients.
2 However, unregulated immune system activation often causes immune-related adverse events (irAEs) and restricts further treatment. The irAEs affect nearly every organ and have a reported incidence of 15% to 90% in cancer patients.
3 While neurologic irAEs reportedly occur less frequently in 1–14% of patients treated using ICIs, they must be recognized due to their potential severity.
4 Here we describe a patient with renal cell carcinoma (RCC) who received a combination of ICIs, nivolumab plus ipilimumab, and developed lumbosacral polyradiculoneuropathy, a rare ICI-related neurologic irAE.
CASE
A 56-year-old female was referred to a neurologist with progressive gait disturbance with pins-and-needles sensations in the feet and below the knee in the legs, which had initially presented 19 days previously. The patient had been diagnosed with metastatic RCC and was being treated using a combination of nivolumab and ipilimumab. After two cycles of combination therapy (nivolumab 3 mg/kg and ipilimumab 1 mg/kg every 3 weeks), sensory symptoms appeared on the soles of both feet and worsened after the third therapy cycle. There were no changes in voiding or bowel patterns. A neurologic examination revealed nearly symmetric weaknesses in both legs, with proximal and distal scores on the Medical Research Council Scale of Muscle Strength of 4.5/5 and 4/5, respectively. Hypoesthesia/paresthesia and decreased vibration sensation were detected below the knees and were more pronounced distally. Deep tendon reflexes were impaired in the right knee and were absent in both ankles, but normoactive at other locations. Babinski signs were absent. Examination results of the cranial nerves and upper extremities were normal.
Nerve conduction study (NCS) results were normal in the upper extremities but revealed abnormalities in the lower extremities, including symmetrically increased terminal latencies, decreased compound motor action potential amplitudes, decreased conduction velocities, and prolonged F-wave latencies in the peroneal and posterior tibial nerves (
Table 1). The sensory responses in bilateral sural nerves were within normal limits. Needle electromyography findings were normal in the lumbosacral paraspinal and lower extremity muscles. These investigations indicated the presence symmetric lumbosacral polyradiculoneuropathy with demyelinating features. Contrast-enhanced magnetic resonance imaging (MRI) of the spine presented enhanced cauda equina with sparing of spinal cord (
Fig. 1). Brain MRI results were normal. Subsequent cerebrospinal fluid (CSF) was negative for malignant cells, but lymphocytic pleocytosis (WBC 120/mm
3; 91% lymphocytes and 9% monocytes), elevated protein (206 mg/dL), and normal glucose levels were observed. Extensive tests excluded infectious and other autoimmune etiologies. CSF staining and culture for bacteria, fungi, and mycobacteria, venereal disease research laboratory tests in the CSF, and CSF polymerase chain reaction for herpes simplex viruses 1 and 2, varicella zoster virus, cytomegalovirus, Epstein-Barr virus, human herpesvirus 6, enterovirus, and
Mycoplasma pneumoniae were all negative. The patient was diagnosed with immunotherapy-related lumbosacral polyradiculoneuropathy in the lower extremities and was treated using 1 g of methylprednisolone each day for 5 days, which improved the sensory symptoms. The patient was discharged on an oral prednisolone taper and the planned third chemotherapy cycle was canceled. After 1 month of initial steroid therapy, the patient regained all strength except for in the toes, and experienced mild numbness and tingling in the feet. In a follow-up NCS, conduction velocities and F-wave latencies of the peroneal and posterior tibial nerves were improved (
Table 1).
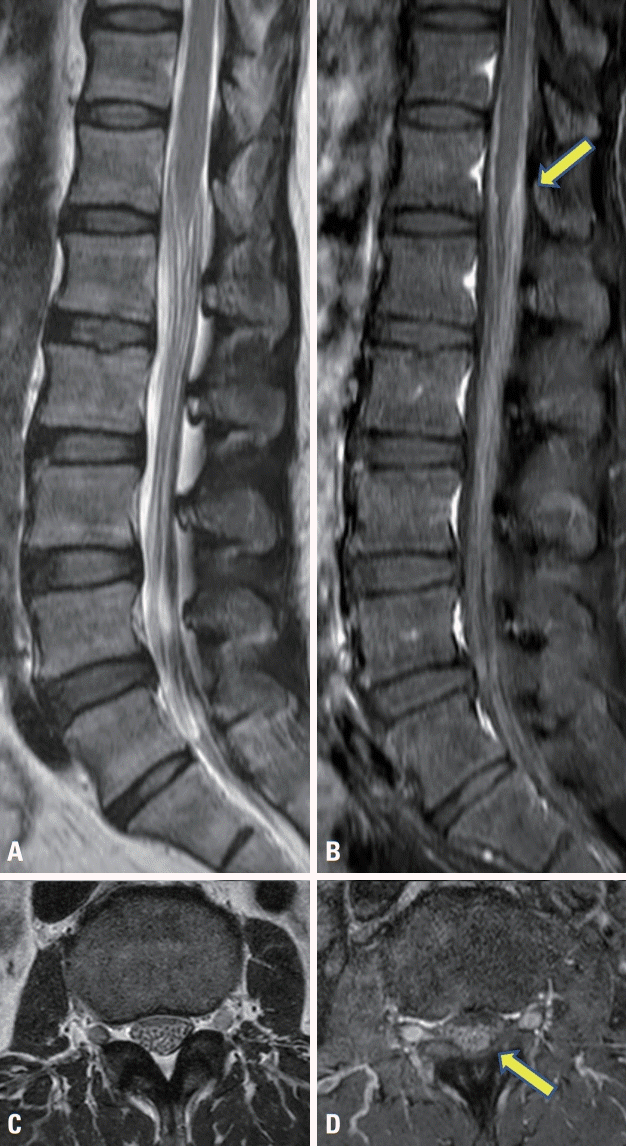 | Fig. 1.Sagittal and axial T2-weighted (A, C) and postcontrast T1-weighted (B, D) magnetic resonance imaging demonstrate thickened and enhanced cauda equina. The enhanced cauda equina is indicated by the arrow (B, D). 
|
Table 1.
Changes in motor NCS findings of the lower extremities before treatment (initial) and 1 month after treatment (follow-up)
|
Motor NCS |
Initial
|
F latency (ms) |
Follow-up
|
F latency (ms) |
|
Latency (ms) |
Amplitude (mV) |
Velocity (m/s) |
Latency (ms) |
Amplitude (mV) |
Velocity (m/s) |
|
Left peroneal |
|
|
|
74.9 |
|
|
|
60.3 |
|
|
EDB-ankle |
4.00 |
1.00 |
|
|
5.05 |
1.04 |
|
|
|
Ankle-head of the fibula |
15.30 |
0.62 |
27.4 |
|
14.40 |
0.84 |
32.6 |
|
|
Right peroneal |
|
|
|
71.2 |
|
|
|
56.3 |
|
EDB-ankle |
4.00 |
0.96 |
|
|
5.08 |
1.14 |
|
|
|
Ankle-head of the fibula |
15.30 |
0.83 |
27.4 |
|
13.50 |
0.99 |
36.2 |
|
|
Left tibial |
|
|
|
71.5 |
|
|
|
57.9 |
|
Abductor hallucis-ankle |
5.19 |
3.1 |
|
|
2.71 |
3.5 |
|
|
|
Ankle-popliteal fossa |
16.40 |
1.64 |
32.1 |
|
13.00 |
2.9 |
31.7 |
|
|
Right tibial |
|
|
|
65.7 |
|
|
|
58.5 |
|
Abductor hallucis-ankle |
4.58 |
4.6 |
|
|
3.78 |
4.3 |
|
|
|
Ankle-popliteal fossa |
14.80 |
2.3 |
32.0 |
|
14.10 |
2.8 |
33.8 |
|

Go to :

DISCUSSION
Currently two immune checkpoints (cytotoxic T lymphocyte-associated antigen-4 [CTLA-4] and programmed cell death protein-1 [PD-1]) are the main targets of ICIs. The CTLA-4 and PD-1 pathways downregulate T-cell activation to maintain peripheral immune tolerance and can be used by cancer cells to avoid immune surveillance.
1 CTLA-4 blockades (e.g., ipilimumab) primarily act early in the immune response of lymph nodes, whereas PD-1 blockades (e.g., nivolumab) primarily act later in the immune response of inflamed peripheral tissues.
1 A combination of ipilimumab and nivolumab enhanced antitumor activity in several tumors including metastatic RCC,
5 but irAEs-related treatment was also highly likely to be discontinued.
6 irAEs commonly occur in the gastrointestinal, hepatic, pulmonary, renal, and endocrine systems, and in the skin.
3 Neurologic irAEs are rare, but they are diverse, including meningitis, encephalitis, myelopathy, and peripheral neuropathies such as Guillain-Barré syndrome (GBS) and myasthenic syndrome.
4 To avoid substantial morbidity and mortality from neurologic irAEs, prompt recognition and treatment are essential.
Here we report the case of a patient who developed a lumbosacral polyradiculoneuropathy at 3 weeks after initiating nivolumab and ipilimumab treatment for metastatic RCC. Ascending paralysis and sensory changes remained in the lower extremities for about 3 weeks. Only a few cases of polyradiculoneuropathy following ICIs have been reported.
7 The most common symptom was symmetrical leg weakness, but the severity has varied. Disease onset varied from 5 to 322 days after the first ICI treatment, and both acute and chronic forms of disease progression were identified. Electrophysiologic studies have often identified demyelination features, and in some cases that received spine MRI, nerve root hypertrophy with contrast enhancement was also observed. However, mild lymphocytic pleocytosis can be found in the CSF, which is not a feature in GBS or chronic inflammatory demyelinating polyneuropathy. In the current case, pleocytosis and protein levels in CSF were higher than in other reports and the symptom severity was rather mild. The correlations among NCS findings, CSF findings, and clinical manifestations still need to be established in neurologic irAEs by analyzing more cases. When first diagnosed, ICIs treatment should not continue in order to minimize further exacerbation. For the current case, there was a favorable therapeutic response to steroids, and intravenous immunoglobulin or plasmapheresis could also be considered in severe cases.
Polyradiculoneuropathy is a clear subset of neurologic irAEs from ICIs. Early recognition and management of neurologic irAEs is important to improve the prognosis. Lumbar puncture and electrodiagnostic studies are necessary when neuropathy is suspected and to rule out alternative diagnoses. Steroids may be the first line treatment for polyradiculoneuropathy induced by ICIs.
Go to :


