This article has been
cited by other articles in ScienceCentral.
Abstract
Facial diplegia (FD) rarely occurs as a regional Guillain-Barré syndrome (GBS) variant. A 70-year-old male presented with bifacial weakness that had started on the left side and extended to the right after several days. He was then treated using steroids and gradually improved. Serum antiganglioside antibody testing revealed positivity for anti-GM1 IgG antibodies. FD can be idiopathic, but it is an uncommon GBS variant. The ganglioside antibody test may increase the possibility of diagnosing isolated FD.
Go to :

Keywords: Facial diplegia, Guillain-Barré syndrome, Facial palsy, Antibodies, Gangliosides, Cranial neuropathy
Unilateral facial nerve palsy is a relatively common neurological disorder with a good prognosis. Facial diplegia (FD) occurs very rarely in facial palsy.
1 Bell’s palsy is the most common cause of unilateral facial palsy, but differential FD diagnoses include Guillain-Barré syndrome (GBS), Lyme disease, brainstem stroke, sarcoidosis, and tuberculous meningitis.
1 Of these, FD can present as a regional GBS variant. The presence of distal paresthesia and pleocytosis can help to make a definitive diagnosis,
1,
2 but few studies have investigated FD without paresthesia.
3 We report an FD case without paresthesia that was initially diagnosed as idiopathic bilateral facial paralysis and then subsequently definitively diagnosed using anti-GM1 antibodies.
CASE
A 70-year-old male presented with sudden left-sided facial palsy that became bilateral despite 2 days of treatment with 60 mg/day prednisolone. On the 7th day after symptom onset, slow-progressing facial weakness was observed, bilateral facial weakness worsened, and it became difficult for the patient to wrinkle, blink, and puff out his cheeks. He had no recent infection history, including upper respiratory infections or gastroenteritis. A neurological examination identified bilateral complete peripheral facial palsy (House-Brackmann grade IV on both sides). Other cranial functions were normal, including extraocular muscle movements. There was no definite weakness or distal paresthesia in the upper and lower extremities. Deep tendon reflexes in the upper and lower extremities were normoactive, and there was no ataxia or gait disturbance.
Laboratory tests revealed no inflammation signs such as leukocytosis and elevations of the C-reactive protein level and erythrocyte sedimentation rate. The serum angiotensin-converting enzyme level and vasculitis screening tests were normal. No paraproteins were detected by serum protein electrophoresis. Serum analysis revealed previous herpes simplex and varicella zoster viral infections, but tests for anti-HSV immunoglobulin (Ig) M and anti-VZV IgM were negative. A cerebrospinal fluid (CSF) examination revealed albuminocytological dissociation (white blood cell count, 1/mm
3; protein, 110.2 mg/dL).
Borrelia burgdorferi antibody testing was not performed. Brain magnetic resonance imaging (MRI) revealed inflammation signs in both facial nerves at the meatal segments (
Fig. 1). Nerve conduction studies (NCSs) performed 7 days after symptom onset including for late responses revealed that the median, ulnar, peroneal, tibial, and sural nerves were normal. Repetitive stimulation tests were negative. Compound muscle action potentials (CMAPs) were reduced in both facial nerves, and distal latency was prolonged in the right facial nerve (
Table 1).
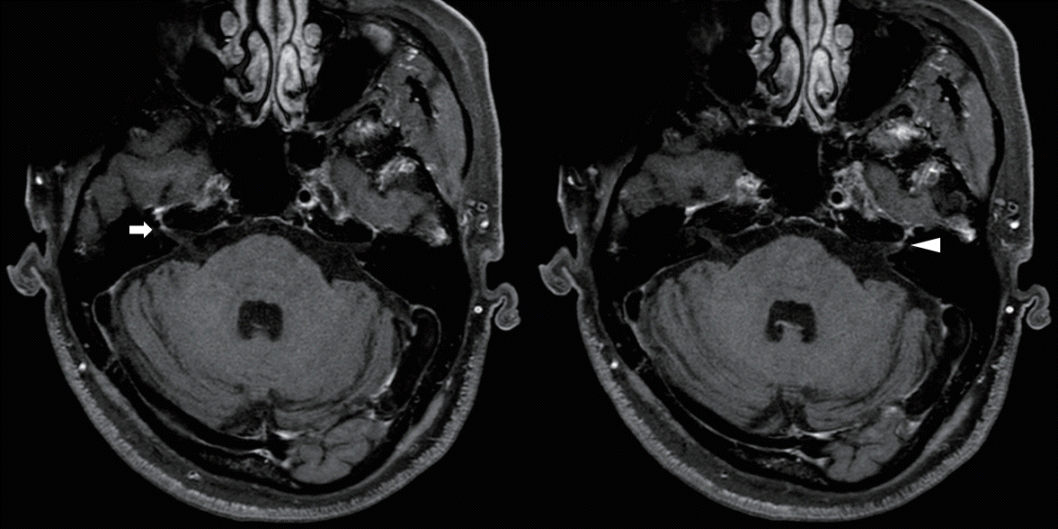 | Fig. 1.Postcontrast three-dimensional T1-weighted magnetic resonance imaging of the brain. Both facial nerves show contrast enhancement at the meatal segments (right, arrow; left, arrowhead). 
|
Table 1.
Facial NCS and blink reflex findings
|
Recording |
Initial |
Follow-up after 4 months
|
Reference values
|
|
Lat. (ms) |
Amp. (mV) |
Lat. (ms) |
Amp. (mV) |
Lat. (ms) |
Amp. (mV) |
|
Facial NCS |
|
|
|
|
|
|
|
|
Lt. O. oculi |
2.4 |
0.8a
|
2.4 |
1.8 |
3.0 |
1.0 |
|
Rt. O. oculi |
3.4a
|
0.3a
|
2.9 |
0.7a
|
3.0 |
1.0 |
|
Blink reflex |
|
|
|
|
|
|
|
Lt. Stim. |
|
|
|
|
|
|
|
R1 Lat. |
NRa
|
|
12.87 |
|
13.0 |
|
|
R2 Ipsi. Lat. |
42.06a
|
|
37.35 |
|
41.0 |
|
|
R2 Cont. Lat. |
40.41 |
|
37.89 |
|
44.0 |
|
|
Rt. Stim. |
|
|
|
|
|
|
|
R1 Lat. |
18.04a
|
|
14.36a
|
|
13.10 |
|
|
R2 Ipsi. Lat. |
36.77 |
|
35.74 |
|
41.0 |
|
|
R2 Cont. Lat. |
NRa
|
|
37.54 |
|
44.0 |
|

Regarding blink reflex, prolonged ipsilateral R1, normal ipsilateral R2, and absent contralateral R2 responses were observed for right supraorbital nerve stimulation (
Table 1). Left supraorbital nerve stimulation revealed that ipsilateral R1, prolonged ipsilateral R2, and normal contralateral R2 responses were absent. Facial NCS and the blink reflex suggested a bilateral facial nerve lesion.
At the previous hospital, prednisolone was administered at 60 mg/day for 2 days to treat the facial palsy, and he was then transferred to our hospital. While taking prednisolone from the previous hospital, the patient subjectively complained of worsening symptoms. For this reason, 1,000 mg of methylprednisolone was intravenously administered for 2 days after admission. Oral prednisolone (60 mg/day) was readministered on a tapered schedule with reduction by 10 mg/day after no clinically significant deterioration was observed. Famciclovir (750 mg/day) was also administered orally for 7 days.
An enzyme-linked immunosorbent assay was performed to detect various antiganglioside antibodies, including IgG and IgM antibodies working against the following gangliosides: GM1, GM2, GD1a, GD1b, GD3, GT1a, GT1b, GQ1b, and GB1b, as described previously.
4 Positivity was found only for anti-GM1 IgG antibodies.
Both facial nerves began recovering after 4 months, at which time the left facial palsy had almost disappeared and the right facial palsy was only mild. During the follow-up on facial NCS and blink reflex after 4 months, we observed a reduced CMAP amplitude in the right facial nerve and prolonged ipsilateral R1 response for right supraorbital nerve stimulation, while other parameters were normal (
Table 1).
Go to :

DISCUSSION
We have described a rare case of isolated FD without a tingling sensation and with a typical CSF composition within GBS. FD with paresthesia (FDP) is a GBS variant. To obtain this diagnosis, other symptoms such as ophthalmoplegia, bulbar symptoms, and cervical or limb weakness should be observed, in addition to limb paresthesia.
5 Other observations supporting an FDP diagnosis include an antecedent infection, albuminocytological dissociation in CSF, and demyelinating neuropathy in the limbs.
5
Since the present patient had no cranial nerve involvement other than facial nerve palsies, limb weakness, or ataxia, it was difficult to diagnose the GBS variant based only on the isolated bilateral facial weakness and albuminocytological dissociation. The abnormal brain MRI and CSF results of this patient were similar to those found in idiopathic Bell’s palsy.
5-
7 Abnormal CSF results have been found in 12.6% of cases of idiopathic Bell’s palsy, including increased protein, and facial nerve enhancement may occur; these findings are therefore not only found in GBS.
5-
7 In such cases it is very important to rapidly identify antiganglioside antibodies for the diagnosis and make treatment decisions for a regional GBS variant.
Nakahara et al.
3 suggested that treatment using intravenous immunoglobulin (IVIG) can be started when GBS is suspected in patients with bilateral facial weakness and abnormal CSF and NCS results. However, in the present patient with idiopathic isolated FD without paresthesia, deciding to treat with IVIG was difficult, and so the patient was treated using steroids for idiopathic facial palsy.
In previous studies, most antiganglioside antibodies were not found in bifacial weakness with paresthesias, and this is known to be caused by demyelinating neuropathy rather than axonal neuropathy.
8-
10 On the other hand, anti-GD1a antibodies have frequently been observed in FD, and there are also rare reports of anti-GM1 IgM or IgG antibodies associated with ophthalmoparesis or lower bulbar palsy.
4,
11 Anti-GD1a antibodies were also recently observed in FDP after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
12 Although anti-GM1 IgG antibodies are mostly associated with acute motor axonal neuropathy, the relationship between IgG anti-GM1 antibodies and FD has not yet been revealed.
4 Since FD can be expressed as an axonal or regional GBS variant, further laboratory tests should be conducted among larger cohorts.
While previous studies have found that using IVIG for FDP is common, few studies have investigated the effects of IVIG in FD without paresthesia.
2,
3,
9 Based on considerable evidence from follow-up studies, IVIG or plasma exchange should be administered in patients with FD with or without paresthesia who are diagnosed with regional GBS variants at an early stage.
In conclusion, this was a very rare case in which anti-GM1 IgG antibodies were detected in FD without paresthesia. In addition to abnormal CSF and brain MRI findings, antiganglioside antibodies can increase the possibility of a regional GBS variant diagnosis. Further studies on idiopathic FD with or without paresthesia are therefore necessary.
Go to :

Notes
Go to :

ACKNOWLEDGMENTS
This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MIST) (No. 2020R1G1A1008446 and 2016R1A5A2007009).
Go to :

REFERENCES
1. Varol S, Ozdemir HH, Akil E, Arslan D, Aluclu MU, Demir CF, et al. Facial diplegia: etiology, clinical manifestations, and diagnostic evaluation. Arq Neuropsiquiatr. 2015; 73:998–1001.

2. Kim JK, Oh SY, Sohn EH, Hong YH, Jun SM, Bae JS. When is facial diplegia regarded as a variant of Guillain-Barré syndrome? J Peripher Nerv Syst. 2015; 20:32–36.

3. Nakahara K, Nakane S, Terasaki T, Ando Y. Effect of phosphatidic acid on antiganglioside antibody reactivity in the isolated facial diplegia variant of Guillain-Barré syndrome: a case report. Acta Neurol Belg. 2021; Jan. 8. [Epub]. DOI:10.1007/s13760-020-01592-z.

4. Kim JK, Bae JS, Kim DS, Kusunoki S, Kim JE, Kim JS, et al. Prevalence of anti-ganglioside antibodies and their clinical correlates with Guillain-Barré syndrome in Korea: a nationwide multicenter study. J Clin Neurol. 2014; 10:94–100.

5. Kinoshita T, Ishii K, Okitsu T, Okudera T, Ogawa T. Facial nerve palsy: evaluation by contrast-enhanced MR imaging. Clin Radiol. 2001; 56:926–932.

6. Kohler A, Chofflon M, Sztajzel R, Magistris MR. Cerebrospinal fluid in acute peripheral facial palsy. J Neurol. 1999; 246:165–169.

7. Ljøstad U, Økstad S, Topstad T, Mygland A, Monstad P. Acute peripheral facial palsy in adults. J Neurol. 2005; 252:672–676.

8. Yuki N, Hartung HP. Guillain-Barré syndrome. N Engl J Med. 2012; 366:2294–2304.

9. Wakerley BR, Yuki N. Isolated facial diplegia in Guillain-Barré syndrome: bifacial weakness with paresthesias. Muscle Nerve. 2015; 52:927–932.

10. Susuki K, Koga M, Hirata K, Isogai E, Yuki N. A Guillain Barré syndrome variant with prominent facial diplegia. J Neurol. 2009; 256:1899–1905.

11. Son H, Kim A, Hong SB, Koo DL. Acute unilateral isolated abducens nerve palsy associated with anti-GM1 immunoglobulin m antibody. Ann Clin Neurophysiol. 2019; 21:105–107.

12. Strawbridge J, Flavin W, Fu K, Chan J, Cohen J, Shieh P, et al. Facial diplegia with paresthesias associated with GD1a antibodies (2955). Neurology. 2021; 96:2955.
Go to :






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download