Abstract
Background
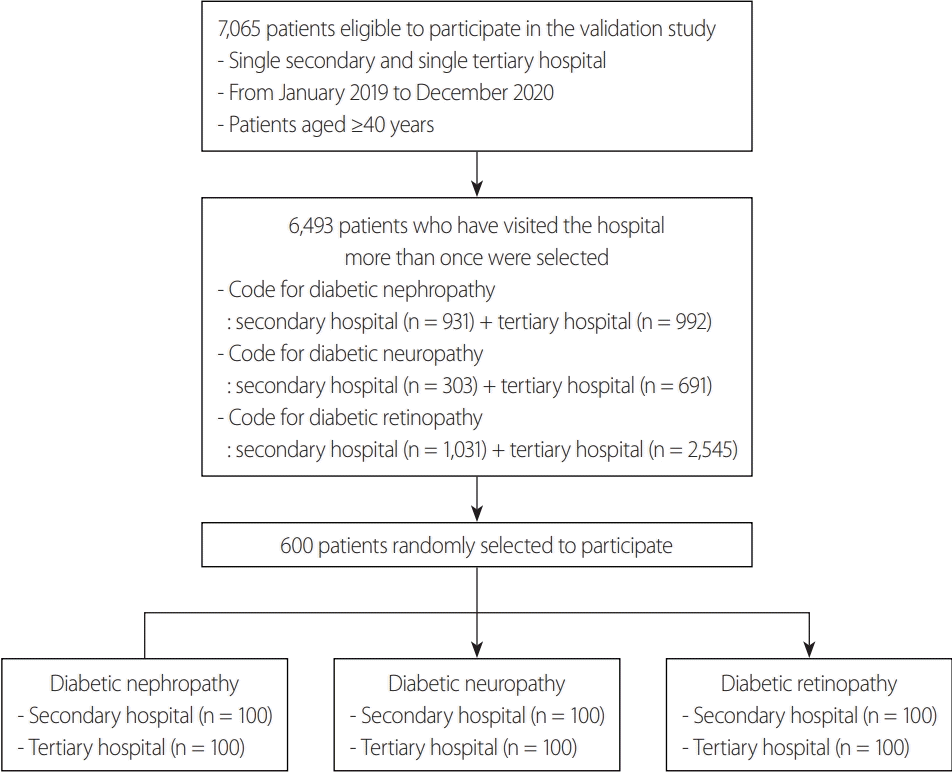
Methods
Results
Supplementary Material
Supplementary Table 1.
Supplementary Table 2.
Supplementary Table 3.
Supplementary Table 4.
REFERENCES
Table 1.
| Total | True | False | PPV (%) | NPV (%) | Error rate (%) | Sensitivity (%) | Specificity (%) | Kappa value | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Secondary hospital | ||||||||||
| Diagnostic codesa (≥ 2 hospital visits) | 100 | 70 | 30 | 70.0 (70.0–70.0) | ||||||
| Diagnostic codesa + blood test | 95 | 69 | 26 | 72.6 (62.5–81.3) | 80.0 (28.4–99.5) | 27.0 (18.6–36.8) | 98.6 (92.3–99.9) | 13.3 (3.8–30.7) | 0.806 | |
| Diagnostic codesa + urinary proteinuria | 75 | 58 | 17 | 77.3 (66.2–86.2) | 52.0 (31.3–72.2) | 29.0 (20.3–38.9) | 82.9 (72.0–90.8) | 43.3 (25.5–62.6) | 0.865 | |
| Diagnostic codesa + blood test + urinary proteinuria | 75 | 58 | 17 | 77.3 (66.2–86.2) | 52.0 (31.3–72.2) | 29.0 (20.3–38.9) | 82.9 (72.0–90.8) | 43.3 (25.5–62.6) | 0.896 | |
| Tertiary hospital | ||||||||||
| Diagnostic codesa (≥ 2 hospital visits) | 100 | 72 | 28 | 72.0 (72.0–72.0) | ||||||
| Diagnostic codesa + blood test | 97 | 71 | 26 | 73.2 (63.2–81.7) | 66.7 (9.4–99.2) | 27.0 (18.6–36.8) | 98.6 (92.5–99.9) | 7.1 (0.9–23.5) | 0.806 | |
| Diagnostic codesa + urinary proteinuria | 71 | 60 | 11 | 84.5 (74.0–92.0) | 58.6 (38.9–76.5) | 23.0 (15.2–32.5) | 83.3 (72.7–91.1) | 60.7 (40.6–78.5) | 0.865 | |
| Diagnostic codesa + blood test + urinary proteinuria | 70 | 60 | 10 | 85.7 (75.3–92.9) | 60.0 (40.6–77.3) | 22.0 (14.3–31.4) | 83.3 (72.7–91.1) | 64.3 (44.1–81.4) | 0.896 | |
| Total | ||||||||||
| Diagnostic codesa (≥ 2 hospital visits) | 200 | 142 | 58 | 71.0 (71.0–71.0) | ||||||
| Diagnostic codesa + blood test | 192b | 140 | 52 | 72.9 (66.1–79.1) | 75.0 (34.9–96.8) | 27.0 (20.9–33.7) | 98.6 (95.0–99.8) | 10.3 (3.9–21.2) | 0.806 | |
| Diagnostic codesa + urinary proteinuria | 146c | 118 | 28 | 80.8 (73.5–86.9) | 55.6 (41.4–69.1) | 26.0 (20.1–32.7) | 83.1 (75.9–88.9) | 51.7 (38.2–65.1) | 0.865 | |
| Diagnostic codesa + blood test + urinary proteinuria | 145d | 118 | 27 | 81.4 (74.1–87.4) | 56.4 (42.3–69.7) | 25.5 (19.6–32.1) | 83.1 (75.9–88.9) | 53.4 (39.9–66.7) | 0.896 | |
Table 2.
| Total | True | False | PPV (%) | NPV (%) | Error rate (%) | Sensitivity (%) | Specificity (%) | Kappa value | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Secondary hospital | ||||||||||
| Diagnostic codesa (≥ 2 hospital visits) | 100 | 59 | 41 | 59.0 (59.0–59.0) | ||||||
| Diagnostic codesa + NCS | 65 | 50 | 15 | 76.9 (64.8–86.5) | 74.3 (56.7–87.5) | 24.0 (16.0–33.6) | 84.8 (73.0–92.8) | 63.4 (46.9–77.9) | 0.878 | |
| Diagnostic codesa + Prescriptionb | 86 | 58 | 28 | 67.4 (56.5–77.2) | 92.9 (66.1–99.8) | 29.0 (20.4–38.9) | 98.3 (90.9–99.9) | 31.7 (18.1–48.1) | 0.912 | |
| Diagnostic codesa + NCS + prescriptionb | 63 | 50 | 13 | 79.4 (67.3–88.5) | 75.7 (58.8–88.2) | 22.0 (14.3–31.4) | 84.8 (73.0–92.8) | 68.3 (51.9–81.9) | 0.951 | |
| Tertiary hospital | ||||||||||
| Diagnostic codesa (≥ 2 hospital visits) | 100 | 51 | 49 | 51.0 (51.0–51.0) | ||||||
| Diagnostic codesa + NCS | 61 | 46 | 15 | 75.4 (62.7–85.5) | 87.2 (72.6–95.7) | 20.0 (12.7–29.2) | 90.2 (78.6–96.7) | 69.4 (54.6–81.8) | 0.878 | |
| Diagnostic codesa + prescriptionb | 70 | 44 | 26 | 62.9 (50.5–74.1) | 76.7 (57.7–90.1) | 33.0 (23.9–43.1) | 86.3 (73.7–94.3) | 46.9 (32.5–61.7) | 0.912 | |
| Diagnostic codesa + NCS + prescriptionb | 49 | 41 | 8 | 83.7 (70.3–92.7) | 80.4 (66.9–90.2) | 18.0 (11.0–27.0) | 80.4 (66.9–90.2) | 83.7 (70.3–92.7) | 0.951 | |
| Total | ||||||||||
| Diagnostic codesa (≥ 2 hospital visits) | 200 | 110 | 90 | 55.0 (55.0–55.0) | ||||||
| Diagnostic codesa + NCS | 126c | 96 | 30 | 76.2 (67.8–83.3) | 81.1 (70.3–89.3) | 22.0 (16.5–28.4) | 87.3 (79.6–92.9) | 66.7 (56.0–76.3) | 0.878 | |
| Diagnostic codesa + prescriptionb | 156d | 102 | 54 | 65.4 (57.4–72.8) | 81.8 (67.3–91.8) | 31.0 (24.7–37.9) | 92.7 (86.2–96.8) | 40.0 (29.8–50.9) | 0.912 | |
| Diagnostic codesa + NCS + prescriptionb | 112e | 91 | 21 | 81.3 (72.8–88.0) | 78.4 (68.4–86.5) | 20.0 (14.7–26.2) | 82.7 (74.4–89.3) | 76.7 (66.6–84.9) | 0.951 | |
Values are presented as number (95% confidence interval).
PPV, positive predictive value; NPV, negative predictive value; NCS, nerve conduction study.
a Visited the hospital more than once with all diagnostic codes (hospital admission + outpatient clinic);
Table 3.
| Total | True | False | PPV (%) | NPV (%) | Error rate (%) | Sensitivity (%) | Specificity (%) | Kappa value | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Secondary hospital | ||||||||||
| Diagnostic codesa (≥ 2 hospital visits) | 100 | 83 | 17 | 83.0 (74.2–89.7) | ||||||
| Diagnostic codesa + fundoscopy | 100 | 83 | 17 | 83.0 (74.2–89.7) | 0.0 (0.0–0.0) | 17.0 (12.6–26.1) | 100.0 (100.0–100.0) | 0.0 (0.0–0.0) | 0.929 | |
| Diagnostic codesa + visit ophthalmologist + fundoscopy | 85 | 79 | 6 | 92.9 (85.3–97.4) | 73.3 (44.9–92.2) | 10.0 (4.9–17.6) | 95.2 (88.1–98.7) | 64.7 (38.3–85.8) | 0.929 | |
| Diagnostic codesa + visit ophthalmologist + fundoscopy + specific treatmentb | 78 | 77 | 1 | 98.7 (93.1–99.9) | 72.7 (49.8–89.3) | 7.0 (2.9–13.9) | 92.8 (84.9–97.3) | 94.1 (71.1–99.9) | 0.964 | |
| Tertiary hospital | ||||||||||
| Diagnostic codesa (≥ 2 hospital visits) | 100 | 71 | 29 | 71.0 (71.0–71.0) | ||||||
| Diagnostic codesa + fundoscopy | 99 | 71 | 28 | 71.7 (61.4–80.1) | 100.0 (100.0–100.0) | 28.0 (19.5–37.9) | 100.0 (100.0–100.0) | 3.4 (0.1–17.8) | 0.929 | |
| Diagnostic codesa + visit ophthalmologist + fundoscopy | 99 | 71 | 28 | 71.7 (61.4–80.1) | 100.0 (100.0–100.0) | 28.0 (19.5–37.9) | 100.0 (100.0–100.0) | 3.4 (0.1–17.8) | 0.929 | |
| Diagnostic codesa + visit ophthalmologist + fundoscopy + specific treatment | 68 | 64 | 4 | 94.1 (85.6–98.4) | 78.1 (60.0–90.7) | 11.0 (5.6–18.8) | 90.1 (80.7–95.9) | 86.2 (68.3–96.1) | 0.964 | |
| Total | ||||||||||
| Diiagnostic codesa (≥ 2 hospital visits) | 200 | 154 | 46 | 77.0 (77.0–77.0) | ||||||
| Diagnostic codesa + fundoscopy | 199c | 154 | 45 | 77.4 (70.8–82.9) | 100.0 (100.0–100.0) | 22.5 (16.5–28.8) | 100.0 (100.0–100.0) | 2.2 (0.1–11.5) | 0.929 | |
| Diagnostic codesa + visit ophthalmologist + fundoscopy | 184d | 150 | 34 | 81.5 (75.2–86.9) | 75.0 (47.6–92.7) | 19.0 (13.8–25.1) | 97.4 (93.5–99.3) | 26.1 (14.3–41.1) | 0.929 | |
| Diagnostic codesa + visit ophthalmologist + fundoscopy + specific treatment | 146e | 141 | 5 | 96.6 (92.2–98.9) | 75.9 (62.4–86.5) | 9.0 (5.4–13.9) | 91.6 (86.0–95.4) | 89.1 (76.4–96.4) | 0.964 | |
Values are presented as number (95% confidence interval).
PPV, positive predictive value; NPV, negative predictive value.
a Visited the hospital more than once with all diagnostic codes (hospital admission + outpatient clinic);
b Prescription (ophthalmic solution with 0.1% bromfenac, calcium dobesilate hydrate, sulodexide, vaccinium myrtillus, and bevacizumab) or retinal photocoagulation;




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download