This article has been
cited by other articles in ScienceCentral.
Abstract
Myocarditis and/or pericarditis have been reported as adverse events following coronavirus disease 2019 (COVID-19) messenger RNA vaccination, with most cases occurring within 1 week after the second dose. We report a rare case of myocarditis after the first dose of the BNT162b2 (Pfizer-BioNTech) COVID-19 vaccine in a 17-year-old boy. Here, we describe the laboratory, electrocardiographic, and imaging findings of myocarditis.
Go to :

Graphical Abstract
Go to :

Keywords: Myocarditis, mRNA Vaccines, Adolescent, COVID-19
INTRODUCTION
According to the Korea Centers for Disease Control and Prevention, for Koreans < 18 years, there were 18.3 cases per 10,000 reports of adverse reactions after coronavirus disease 2019 (COVID-19) vaccination.
1 The most common side effects of messenger RNA vaccination are injection site pain, headache, myalgia, chills, and fever
2; myocarditis as a side effect is rare in adolescents and young adults and is more common in males.
34 The rate of myocarditis/pericarditis after the second dose of vaccination among Koreans 12–39 years of age is approximately 12.6 cases per million.
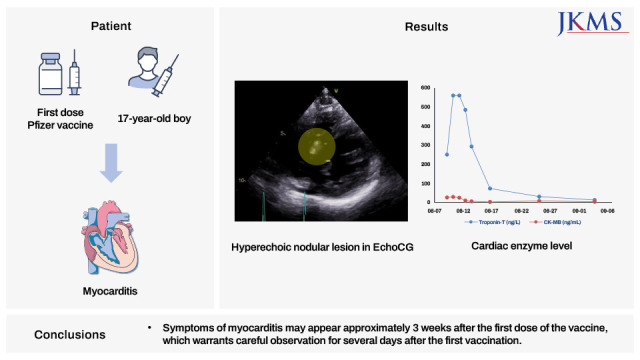
5 Most complaints of post-vaccination myocarditis symptoms occur within 5 days of administration of the second dose. Here, we report a case of myocarditis presenting with a hyperechoic nodule in a 17-year-old boy that occurred 18 days after receiving the first dose of BNT162b2 (Pfizer-BioNTech) vaccine.
Go to :

CASE DESCRIPTION
A 17-year-old boy visited the emergency room complaining of chest pain that first occurred the night before. He described the pain as pressure on the middle of the chest and mild on a numerical rating scale of 2. However, he did not complain of fever, nausea, vomiting, or dyspnea. The patient had no history of COVID-19 or any other relevant medical history. He had received the first dose of the BNT162b2 COVID-19 vaccine 18 days prior to the onset of chest pain. No discomfort was noted during inoculation.
His vital signs at the time of admission were as follows: body temperature, 36.4 °C; blood pressure, 104/58 mmHg; and pulse rate, 78 beats per minute. His height, weight, and body mass index were 174.7 cm (75th percentile), 60 kg (25th percentile), and 20.2 kg/m
2 (25th percentile), respectively. On physical examination, lung sounds were clear, heartbeats were regular, and there was no cardiac murmur. Findings of the blood tests performed at admission were as follows: white blood cell count, 6,120/μL; C-reactive protein, 16.93 mg/L; Troponin-T, 0.251 ng/mL (range, 0–0.1); and creatine kinase-myocardial band, 26.07 ng/mL (range, 0–4.94). Serological viral markers, including rubella, Epstein-Barr virus, and cytomegalovirus, were negative. Polymerase chain reaction testing for common respiratory viruses, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was negative. Antinuclear antibody and rheumatoid factor tests for autoimmune diseases were negative. Chest radiography revealed no abnormal findings, including cardiomegaly. An electrocardiogram on admission showed diffuse ST-segment elevation in leads II, III, aVF, V3, V4, V5, and V6 (
Fig. 1). Echocardiography revealed a hyperechoic nodular lesion in the right ventricular side of the interventricular septal wall (
Fig. 2A and B). The estimated ejection fraction in the M-mode was normal (65.8%). As ST elevation on the electrocardiogram in leads II, III, aVF, V3, V4, V5, V6, and elevation of cardiac biomarkers were detected, a cardiac computed tomographic (CT) angiography was performed to exclude myocardial infarction, and this showed no evidence of coronary artery disease. Additionally, cardiac magnetic resonance imaging (MRI) was performed to differentiate between focal myocardial infarction and myocarditis. We use a 3.0 Tesla MRI machine (Siemens). The MRI showed late gadolinium enhancement in the mid-base septum and apex lateral wall (
Fig. 3). The T1 and T2 weighted images were normal, and there was no evidence of edema. Based on the results, acute multifocal myocarditis was diagnosed.
6
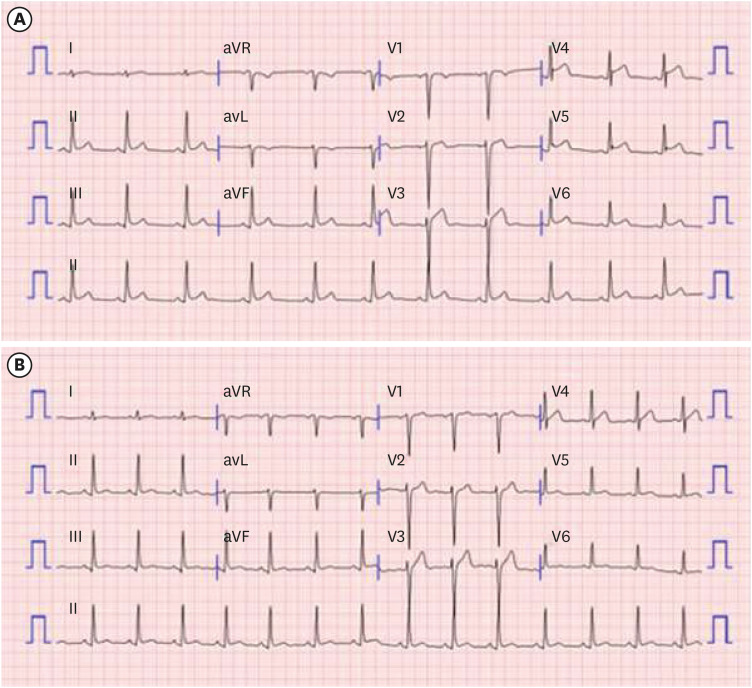 | Fig. 1 Electrocardiography of the patient. (A) At the day of admission, ST-segment elevation was observed in leads II, III, avF, V3, V4, V5 and V6. (B) On hospital day 5, ST-segment elevation improved.
|
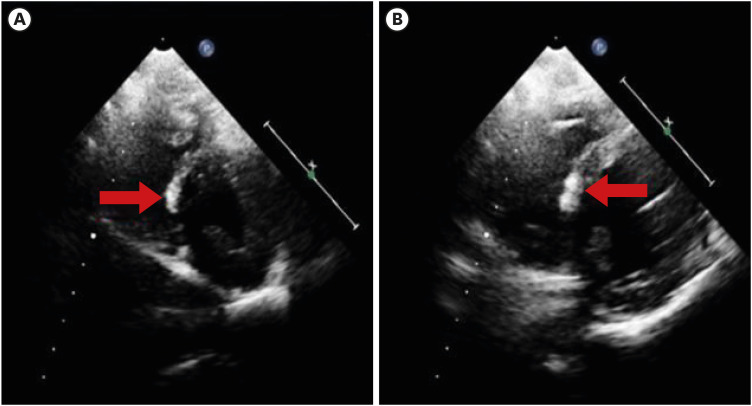 | Fig. 2 Echocardiography on hospital day 2 showed a hyperechoic nodular lesion in the right ventricular side of the interventricular septal wall (A, short axis apex view; B, Short axis mid view).
|
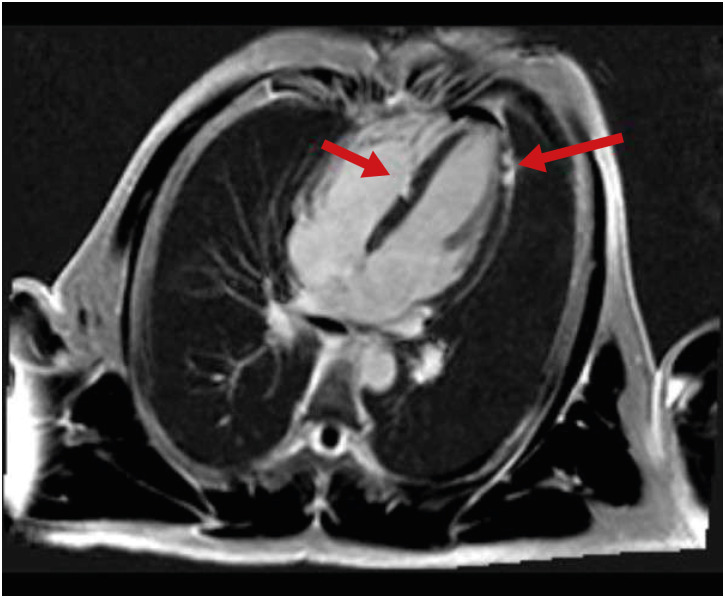 | Fig. 3 Cardiac magnetic resonance imaging of the patient was conducted on hospital day 4. Focal nodular late gadolinium enhancements in the mid-base septum (left arrow) and apex lateral wall (right arrow) are shown.
|
After admission, systolic blood pressure was > 130 mmHg, and chest discomfort was observed. We administered an angiotensin-converting enzyme inhibitor (ACEi, enalapril) 7.5 mg/day for 3 days and then maintained 5 mg/day to control blood pressure. Then, the patient did not complain of chest discomfort, and the systolic pressure was controlled to < 130 mmHg. The possibility of small focal myocardial infarction and accompanying acute myocarditis could not be completely ruled out; therefore, low-dose aspirin 5 mg/kg/day was administered from hospital day 2. Electrocardiogram revealed improved ST-segment elevation on hospital day 5. Troponin-T and creatine kinase-myocardial band peaked at 0.560 ng/mL and 24.98 ng/mL, respectively, on hospital day 3 (21 days after the vaccination) and normalized after hospital day 5. Since all the symptoms resolved and cardiac markers and electrocardiogram normalized on hospital day 8, the patient was discharged.
Ethics statement
The Institutional Review Board of Jeonbuk National University Hospital approved this study (IRB No. 2021-10-024). Informed consent was waived because of the retrospective nature of the study.
Go to :

DISCUSSION
We describe myocarditis in a 17-year-old boy approximately 3 weeks after the first BNT162b2 vaccine dose. The patient was treated with ACEi and aspirin and discharged after the symptoms improved under supportive care.
According to case reports of myocarditis after COVID-19 vaccination, the most common symptom was chest pain.
7 Blood tests showed elevated troponin levels, and electrocardiography showed diffuse ST-segment elevation. In echocardiography, ejection fraction was mostly normal. Cardiac MRI findings were also reviewed.
78
Cardiac MRI is commonly used to diagnose myocarditis based on the Lake Louise criteria.
9 According to the 2018 Lake Louise criteria, in order to diagnose acute myocarditis, at least one of the T1-based criteria and one of the T2-based criteria must be satisfied. In our case, cardiac MRI showed late gadolinium enhancement. But there were no increased T2 relaxation time (44.1 msec), myocardial edema, and no signal change in T2 weighted images. Although this did not meet the Lake Louise criteria, the authors found that most of the cases with myocarditis-related COVID-19 vaccine did not have T2 changes on cardiac MRI.
8 We consider this to be the difference between the MRI characteristics of common myocarditis and myocarditis-related COVID-19 vaccine.
We considered two possibilities for hyperechoic lesions. One possibility is that the hyperechoic lesion was the result of calcification and necrosis in acute myocarditis after the mRNA vaccine. A case of hyperechoic lesions on echocardiography concomitant with late gadolinium enhancement on cardiac MRI was reported by Misumi et al.
10 However, in that case, late gadolinium enhancement on MRI revealed diffuse mid-layer wall striae, suggesting massive left ventricular fibrosis. In our case, the locations of a hyperechoic nodule of echocardiography and late gadolinium enhancement nodule on MRI on ventricular septum coincided. In another, a case of myocardial calcification and confluent myofiber necrosis presented as multiple hyperechogenic foci in acute neonatal myocarditis was reported.
11 Although there was no MRI result in the report, the presentation of echocardiography was quite similar to our case. This will be clear when the changes in the hyperechoic nodule are observed during follow-up. The other possibility is that the hyperechoic lesion was highly fibrous. We found no calcification lesions on the chest X-ray and cardiac CT. Cardiac MRI showed native T1 augmentation and late gadolinium enhancement, suggesting the possibility of a fibrous lesion.
12 To the best of the knowledge of the authors, no cases of acute myocarditis after the mRNA vaccine with hyperechoic nodules in the same lesion as the nodule with late gadolinium enhancement have been reported previously.
It has been reported that most cases of myocarditis after messenger RNA vaccination occur after a second vaccination dose.
513 Symptoms usually appear within 2–3 days after receiving the second dose.
513 Myocarditis following COVID-19 messenger RNA vaccination is more common in young men than in other populations.
14 However, the cause of myocarditis following vaccination has not been clearly established. Two potential mechanisms of COVID-19 vaccine-induced myocarditis have been suggested.
5 One theory concerns the cytokine response, whereby the vaccine-induced immune response triggers an unexpected immune reaction, exacerbating inflammation. Another theory concerns autoantibody reactions; the COVID-19 vaccine can cause myocarditis by producing autoantibodies that affect the function and inflammatory reaction of cardiac myocytes.
10
In our case, myocarditis developed within approximately 3 weeks after the first dose of the COVID-19 messenger RNA vaccine. This was longer than what is usually observed in patients. One hypothesis for this difference is the time interval between the first and second doses of the vaccine. In general, there is a 3-week interval between the administration of the first and second doses.
15 Comparably, in our patient, the time taken to develop acute myocarditis after the first vaccination dose was approximately 3 weeks. Another hypothesis is the possibility that the diagnosis of this event was not myocarditis. The possibility of a small myocardial infarction still exists. However, we did not find any evidence of myocardial infarction. The final hypothesis is that the development of myocarditis occurred in the past before the diagnosis. In our patient, there was no concomitant edematous change in the myocardium around the late gadolinium enhancement area.
In summary, there have been several reports of myocarditis following the administration of the COVID-19 messenger RNA vaccine. We have reported a case of acute myocarditis with a hyperechoic nodular lesion on echocardiography. Even though ventricular function is preserved, late gadolinium enhancement along with hyperechoic nodular lesion can be observed in acute myocarditis due to the mRNA vaccine. Symptoms of myocarditis may appear approximately 3 weeks after the first dose of the vaccine, which warrants careful observation for several days after the first vaccination. If symptoms such as chest pain occur within a few days, it may be a side effect of the vaccine, and a hospital visit may be needed even up to 3 weeks from the day of vaccination.
Go to :