Abstract
Tofacitinib is an oral, small-molecule Janus kinase inhibitor approved in South Korea for the treatment of moderate to severe ulcerative colitis (UC) on May 1, 2019. However, safety data are lacking. We investigated the incidence of serious adverse events (SAEs) in patients with UC using tofacitinib from the National Health Insurance Service database. In all, 1,026 UC patients were enrolled in this study. The overall incidences (100 person-years; 95% confidence interval) of SAEs were 4.06 (1.63–8.36) and 6.30 (4.59–8.43) in the tofacitinib and anti-TNFi groups, respectively. No thromboembolic event occurred and major cardiovascular events occurred in only three patients (two unstable angina and one congestive heart failure) in the tofacitinib group. The incidence of herpes zoster and tuberculosis did not differ between the two groups. There was no difference in the overall incidence of SAEs, including thromboembolic events, between tofacitinib- and TNFi-treated UC patients.
Graphical Abstract
The number of patients with ulcerative colitis (UC) has increased recently in South Korea and other Asian countries, where the disorder was once considered rare.123 Treatment of moderate to severe UC is based on immunosuppression using compounds such as steroids, immunomodulators, and biologics. Tofacitinib is an oral Janus kinase (JAK) inhibitor authorized for the treatment of UC. The efficacy and safety of tofacitinib in adults with moderate to severe UC have been evaluated in clinical studies.45 Tofacitinib was approved for such patients in South Korea on May 1, 2019. However, it has several safety issues. Several adverse events of interest have been reported: infection (severe or opportunistic; herpes zoster [HZ]), major adverse cardiovascular events, malignancy, thromboembolic events.6 The U.S. Food and Drug Administration announced a warning about the increased risk for serious heart-related events, cancer, blood clots and death for JAK inhibitors for the treatment of chronic inflammatory conditions, including UC, on September 1, 2021. UC is an independent risk factor for venous thrombosis.789 The incidence of venous thrombosis in an Asian population was lower compared to a Western population.101112 Therefore, there is a need for research on JAK inhibitor-related thromboembolic events in Asians. Tuberculosis (TB) is another important issue in South Korea because of its high prevalence.13 In one study, active TB associated with tofacitinib was reported in 0.5% of rheumatic arthritis (RA) patients, most of them in epidemic areas.14
We evaluated the safety of tofacitinib in moderate to severe UC by analyzing the National Health Insurance Service (NHIS) database. We used the diagnostic codes defined by the International Classification of Diseases, tenth revision (ICD-10) and the V code in the rare intractable diseases database.15
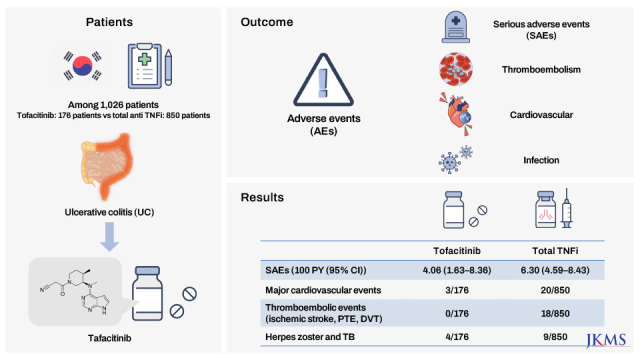
We enrolled UC patients identified by ICD-10 (K51.x) and V code (V131) from the NHIS database for May 1, 2019, to April 30, 2021. Tofacitinib was approved for the treatment of moderate to severe UC in Korea on May 1, 2019. To identify such patients, eligible patients were restricted to those prescribed an anti-TNF inhibitor (infliximab, adalimumab, golimumab) or tofacitinib. We also excluded those aged < 19 years; individuals with previous exposure to biologics or JAK inhibitors; and those with a previous diagnosis of major cardiovascular events (unstable angina [UA], acute myocardial infarction [AMI], congestive heart failure [CHF]), other thromboembolic events (ischemic stroke, pulmonary thromboembolism [PTE], deep vein thrombosis [DVT]), HZ, TB, or cancer (Fig. 1).
Demographic features (age, sex, duration of disease), comorbidities, and UC medication use were collected. Comorbidities included hypertension (ICD-10 code, I10.x–I15.x), diabetes mellitus (E10.x–E14.x), chronic renal failure (N18.x), and liver cirrhosis (K74.x). We also collected data on UC medications, including 5-aminosalicylic acid, immunomodulators (azathioprine, mercaptopurine, cyclosporine, tacrolimus, and methotrexate), steroids, biologics (infliximab, adalimumab, golimumab, vedolizumab), and JAK inhibitors.
Patients were divided into tofacitinib and anti-TNFi groups, and we estimated the incidences of serious adverse events (SAEs) in the two groups. SAEs were defined as major cardiovascular events, other thromboembolic events, HZ, and TB. Major cardiovascular events included UA (I20.0, hospitalization/outpatient), AMI (I21.x, hospitalization), and CHF (I50.x, hospitalization/outpatient). Other thromboembolic events included ischemic stroke (I63.x–I64.x, hospitalization), PTE (I26.x, hospitalization/outpatient) and DVT (I80.x, hospitalization/outpatient). HZ was detected using ICD-10 codes and based on medication use (B02.x and antiviral agents, respectively). TB was detected based on the V code (V000, V206, V246) and use of anti-TB medications.
We reviewed a total of 94,838 UC patients from May 1, 2019 to April 30, 2021. Of these, 1,026 UC patients were enrolled and analyzed in this study (Fig. 1). The tofacitinib and anti-TNFi groups included 176 (17.2%) and 850 (82.8%) UC patients, respectively. The anti-TNFi group was subdivided into infliximab, adalimumab, and golimumab groups (473 [46.1%] vs. 295 [28.7%] vs. 82 [8.0%]). The mean patient age did not differ between the two groups (40.5 ± 14.6 vs. 40.7 ± 14.6 years, P = 0.884). The disease duration was longer in the tofacitinib group than the anti-TNFi group (P < 0.001). Overall comorbidity and medication history did not differ between the two groups.
The overall incidences (100 person-years; 95% confidence interval) of SAEs were 4.06 (1.63–8.36) and 6.30 (4.59–8.43) in the tofacitinib and anti-TNFi groups, respectively. In the tofacitinib group, major cardiovascular events occurred in three patients (two UA and one CHF). CHF was more common in the anti-TNFi group, but there was no statistical significance. No thromboembolic event, such as ischemic stroke, PTE, or DVT, occurred in the tofacitinib group. PTE and DVT occurred in 6 (0.7%) and 12 (1.4%) patients, respectively, in the anti-TNFi group. The incidence of HZ and TB did not differ between the two groups (Table 1).
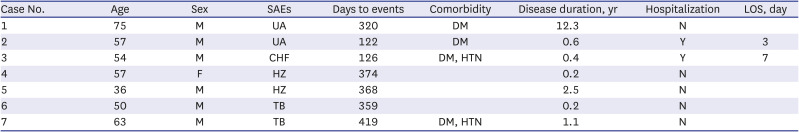
Three patients with major cardiovascular events had DM and one patient had DM and hypertension. Of them, two patients were hospitalized for 3 and 7 days, respectively. The numbers of days to major cardiovascular events were 320, 122, and 126. HZ and TB occurred in four patients, and the average number of days to the events was 380. (Table 2).
There was no difference in the incidences of major cardiovascular events between the tofacitinib and anti-TNFi groups. There were 20 major adverse cardiovascular events among patients receiving any TNFi, for an incidence of 0.51 per 100 person-years in the UC trial-like cohort, compared with 0.28 per 100 person-years (seven patients with major cardiovascular events) in the tofacitinib UC clinical trial overall cohort.16 In a recent study of RA patients, the rate of major adverse cardiovascular events was 0.4 per 100 person-years.14 Our results are similar to those findings. There were two patients with UA and one with CHF among 176 patients in the tofacitinib group. Interestingly, CHF was more common in the anti-TNFi group than the tofacitinib group, albeit the difference was not statistically significantly. An association between anti-TNF agents, such as infliximab and adalimumab, and CHF has long been established.17 Our findings support that notion. In addition, the three patients with major cardiovascular events had DM and were older than the mean age of the study population. Therefore, we suggest caution when using tofacitinib in elderly and diabetic patients.
Pulmonary embolism and DVT, now the most important issue related to tofacitinib, were not found in this study; also, there were no cases of ischemic stroke. These findings do not correspond with recent reports of thromboembolic events in moderate to severe UC patients using tofacitinib.1819 However, a recent study that used claims data reported no pulmonary embolism or DVT but their rates were numerically higher in TNFi-treated patients, as in this study.16 The discrepancy may have one or more of several possible explanations. First, tofacitinib was approved recently for the treatment of moderate to severe UC. Therefore, it is possible that the observation period was too short to confirm the occurrence of thromboembolic events. Second, UC is an independent risk factor for venous thrombosis, the incidence of which in an Asian population was lower compared to a Western population. Third, there was no analysis of patients treated with both tofacitinib and TNFi. This study also had strengths. This is the first comparative study of tofacitinib-related SAEs in South Korea. More importantly, we enrolled only biologic-naïve patients to minimize the impact of previous use of biologics.
The HZ and TB rates were not different between the tofacitinib and anti-TNFi groups. This finding for HZ is similar to other clinical trials, in which the safety of tofacitinib was similar to that of TNFis.620212223 The incidence of TB was numerically low, although TB has a high prevalence in South Korea. Therefore, a latent TB test should be conducted before using tofacitinib or TNFis in South Korea; if the result is negative, those agents can be used.
This study had a major limitation. Although we estimated the person-year data of the incidence of overall SAEs, we could not do so for the person-year data of the incidence of each SAE, because there were no thromboembolic events in the tofacitinib group and a small number of adverse events (HZ, TB).
In conclusion, there was no difference in the overall incidence of SAEs, including thromboembolic events, between tofacitinib- and TNFi-treated UC patients. Therefore, tofacitinib can be used for moderate to severe UC with a similar risk profile to TNFis. However, the follow-up duration was insufficient to evaluate the incidences of SAEs. Collection and analysis of further data are necessary.
Notes
References
1. Lee JY, Oh K, Hong HS, Kim K, Hong SW, Park JH, et al. Risk and characteristics of tuberculosis after anti-tumor necrosis factor therapy for inflammatory bowel disease: a hospital-based cohort study from Korea. BMC Gastroenterol. 2021; 21(1):390. PMID: 34670529.
2. Yen HH, Weng MT, Tung CC, Wang YT, Chang YT, Chang CH, et al. Epidemiological trend in inflammatory bowel disease in Taiwan from 2001 to 2015: a nationwide populationbased study. Intest Res. 2019; 17(1):54–62. PMID: 30449079.
3. Hong SW, Ye BD. The first step to unveil the epidemiology of inflammatory bowel disease in Central Asia. Intest Res. 2020; 18(4):345–346. PMID: 33131230.
4. Sandborn WJ, Ghosh S, Panes J, Vranic I, Su C, Rousell S, et al. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012; 367(7):616–624. PMID: 22894574.
5. Sandborn WJ, Su C, Sands BE, D’Haens GR, Vermeire S, Schreiber S, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017; 376(18):1723–1736. PMID: 28467869.
6. Sandborn WJ, Panés J, D’Haens GR, Sands BE, Su C, Moscariello M, et al. Safety of tofacitinib for treatment of ulcerative colitis, based on 4.4 years of data from global clinical trials. Clin Gastroenterol Hepatol. 2019; 17(8):1541–1550. PMID: 30476584.
7. Grainge MJ, West J, Card TR. Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. Lancet. 2010; 375(9715):657–663. PMID: 20149425.
8. Kappelman MD, Horvath-Puho E, Sandler RS, Rubin DT, Ullman TA, Pedersen L, et al. Thromboembolic risk among Danish children and adults with inflammatory bowel diseases: a population-based nationwide study. Gut. 2011; 60(7):937–943. PMID: 21339206.
9. Nguyen GC, Bernstein CN, Bitton A, Chan AK, Griffiths AM, Leontiadis GI, et al. Consensus statements on the risk, prevention, and treatment of venous thromboembolism in inflammatory bowel disease: Canadian Association of Gastroenterology. Gastroenterology. 2014; 146(3):835–848.e6. PMID: 24462530.
10. Chung WS, Lin CL, Hsu WH, Kao CH. Inflammatory bowel disease increases the risks of deep vein thrombosis and pulmonary embolism in the hospitalized patients: a nationwide cohort study. Thromb Res. 2015; 135(3):492–496. PMID: 25596768.
11. Weng MT, Park SH, Matsuoka K, Tung CC, Lee JY, Chang CH, et al. Incidence and risk factor analysis of thromboembolic events in East Asian patients with inflammatory bowel disease, a multinational collaborative study. Inflamm Bowel Dis. 2018; 24(8):1791–1800. PMID: 29726897.
12. Liu J, Gao X, Chen Y, Mei Q, Zhu L, Qian J, et al. Incidence and risk factors for venous thrombosis among patients with inflammatory bowel disease in China: a multicenter retrospective study. Intest Res. 2021; 19(3):313–322. PMID: 33232589.
13. Jeon D. Latent tuberculosis infection: recent progress and challenges in South Korea. Korean J Intern Med. 2020; 35(2):269–275. PMID: 32131570.
14. Cohen SB, Tanaka Y, Mariette X, Curtis JR, Lee EB, Nash P, et al. Long-term safety of tofacitinib up to 9.5 years: a comprehensive integrated analysis of the rheumatoid arthritis clinical development programme. RMD Open. 2020; 6(3):e001395. PMID: 33127856.
15. Kim HJ, Hann HJ, Hong SN, Kim KH, Ahn IM, Song JY, et al. Incidence and natural course of inflammatory bowel disease in Korea, 2006-2012: a nationwide population-based study. Inflamm Bowel Dis. 2015; 21(3):623–630. PMID: 25647154.
16. Curtis JR, Regueiro M, Yun H, Su C, DiBonaventura M, Lawendy N, et al. Tofacitinib treatment safety in moderate to severe ulcerative colitis: comparison of observational population cohort data from the IBM MarketScan® administrative claims database with tofacitinib trial data. Inflamm Bowel Dis. 2021; 27(9):1394–1408. PMID: 33324993.
17. Sinagra E, Perricone G, Romano C, Cottone M. Heart failure and anti tumor necrosis factor-alpha in systemic chronic inflammatory diseases. Eur J Intern Med. 2013; 24(5):385–392. PMID: 23333028.
18. Sandborn WJ, Panés J, Sands BE, Reinisch W, Su C, Lawendy N, et al. Venous thromboembolic events in the tofacitinib ulcerative colitis clinical development programme. Aliment Pharmacol Ther. 2019; 50(10):1068–1076. PMID: 31599001.
19. Deepak P, Alayo QA, Khatiwada A, Lin B, Fenster M, Dimopoulos C, et al. Safety of tofacitinib in a real-world cohort of patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2021; 19(8):1592–1601.e3. PMID: 32629130.
20. Sandborn WJ, van Assche G, Reinisch W, Colombel JF, D’Haens G, Wolf DC, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012; 142(2):257–265.e1-3. PMID: 22062358.
21. Sandborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, Johanns J, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014; 146(1):85–95. PMID: 23735746.
22. Sandborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, Johanns J, et al. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014; 146(1):96–109.e1. PMID: 23770005.
23. Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005; 353(23):2462–2476. PMID: 16339095.
Fig. 1
Flow diagram of the present study.
UC = ulcerative colitis, TE = thromboembolism, HZ = herpes zoster, TB = tuberculosis.
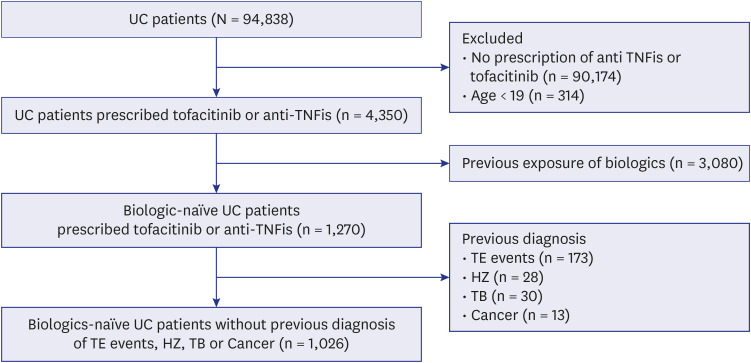
Table 1
Baseline characteristics and SAEs of the study subjects
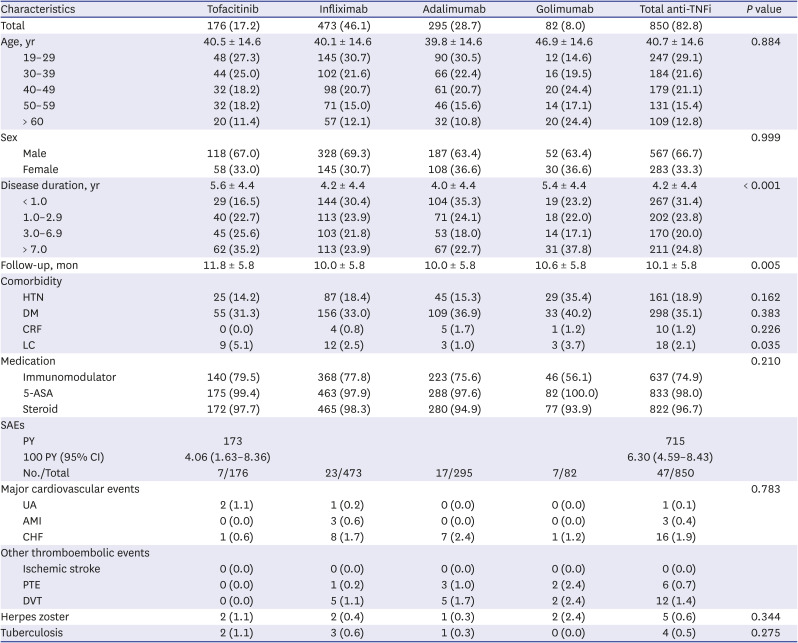
SAE = serious adverse event, HTN = hypertension, DM = diabetes mellitus, CRF = chronic renal failure, LC = liver cirrhosis, 5-ASA = 5-aminosalicylic acid, UA = unstable angina, AMI = acute myocardial infarction, CHF = congestive heart failure, PTE = pulmonary thromboembolism, DVT = deep vein thrombosis, PY = person-year, CI = confidence interval.
Table 2
Case summary of SAEs in tofacitinib




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download