INTRODUCTION
Systemic transrectal ultrasound (TRUS)-guided prostate biopsy has been the standard modality for diagnosing prostate cancer since its introduction by Hodge et al.
1 in 1989. The low sensitivity in detecting clinically significant prostate cancer, a major limitation of TRUS-guided prostate biopsy, and the advent of multiparametric magnetic resonance imaging (MRI) has led to increased utilization of MRI for prostate biopsy in the past decade.
2 While studies have shown higher detection rate of clinically significant prostate cancer for MRI-guided prostate biopsy compared to TRUS-guided biopsy, limitations such as need for significant expertise, financial investment, and the lack of a standardized technique (cognitive or fusion, transrectal or transperineal, number of biopsy cores, etc.) have hindered its widespread use.
3 Therefore, despite its inherent limitations, TRUS-guided prostate biopsy is still the most common method for diagnosing prostate cancer.
4
Various methods of anesthesia have been found to alleviate the pain and discomfort that inevitably accompanies TRUS-guided prostate biopsy.
5 Local anesthesia such as periprostatic lidocaine injection or intrarectal lidocaine instillation is the most commonly used method for TRUS-guided prostate biopsy, both of which can be performed in outpatient settings with relatively fewer resource requirements.
6 Sedation anesthesia with intravenous (IV) agents such as midazolam or propofol has also been studied. While it requires more resources and cost, it was effective in reducing patient discomfort, with the added benefit of significantly more patients willing to undergo re-biopsy, presumably owing to its anxiolytic and amnestic effects.
78 Surprisingly, little is known whether the main clinical outcomes of prostate biopsy such as cancer detection and complication rates can vary with the type of anesthesia. It is known in other fields that important clinical outcomes can be influenced by the type of anesthesia.
910 If clinical outcomes of TRUS-guided prostate biopsy vary depending on type of anesthesia, the decision to choose a certain type of anesthesia should not be based solely on degree of analgesia.
Propofol is a widely used agent for sedation anesthesia in various procedures and minor operations, including prostate biopsy.
1112 It has been used since September 2013 in our institution; prior to that, local anesthesia with intrarectal lidocaine gel instillation was used. The current study aimed to determine whether sedation anesthesia with IV propofol had any effect on cancer detection and complication rates after TRUS-guided prostate biopsy.
METHODS
Study population and design
A retrospective design was used in the current study. A chart review of all patients who underwent TRUS-guided prostate biopsy between November 2009 and February 2019 was performed. Patients who underwent other procedures simultaneously and those who did not have records of at least a 1-month follow-up period were excluded. Our institution had been performing TRUS-guided prostate biopsy using only local anesthesia with intrarectal lidocaine gel instillation until September 2013, from which a complete transition to sedation anesthesia with IV propofol was undertaken. Accordingly, the patients were divided into two groups: patients who underwent sedation anesthesia with IV propofol (sedation group) and patients who underwent local anesthesia with intrarectal lidocaine gel instillation (local group). The following information was collected: age (years), history of disorders that may have caused neurologic bladder dysfunction (diabetes mellitus, stroke, Parkinson’s disease, dementia, spine surgery, pelvic surgery, etc.), history of taking 5-alpha reductase inhibitors prior to biopsy, prostate specific antigen (PSA, ng/mL) level, prostate size (mL), PSA density (ng/mL2), core length (cm) and complications. Complications were classified with the modified Clavien-Dindo classification system. Additional analysis of three major complications (voiding dysfunction, bleeding and infection) was done. These complications were defined as any condition serious enough to alter the normal clinical course such as admission or additional visits to the outpatient clinic. Self-limiting symptoms such as transient voiding difficulty, hematuria, hematospermia, and dysuria that did not alter the normal clinical course, were not included in the analysis.
TRUS-guided prostate biopsy either with local anesthesia with intrarectal lidocaine gel instillation or sedation anesthesia with IV propofol
All patients were administered a single dose of second-generation cephalosporin flomoxef 1 g prior to the procedure. With the patient in the left lateral position, a standard 12-core TRUS-guided prostate biopsy was performed. In the local group, 10 mL of 2% lidocaine gel was instilled into the rectum at least 5 minutes before the procedure. In the sedation group, the attending anesthesiologist conducted a thorough preliminary evaluation including history, physical examination and airway evaluation to determine the risk of sedation anesthesia. The patient was required to fast for at least 6 hours after a light meal and 8 hours after a heavy meal. After preoxygenation, 0.2 mg of glycopyrrolate was administered at approximately 20 minutes before propofol infusion. To prevent pain, 25 mg of pethidine was administered intravenously. An IV bolus injection of 1.5 to 2 mg/kg of propofol was conducted. When the patient was sufficiently sedated, an oropharyngeal airway device was inserted. The patient’s blood pressure, heart rate, respiratory rate, oxygen saturation, and cardiac rhythm were monitored during and after the procedure. Recovery was assessed using the Modified Aldrete Score.
13 The patient was discharged when the total score was 9 or more and the breathing score was 2 or more. All patients were discharged with alpha blockers.
Statistical analysis
Descriptive statistics were used to describe the results. Comparison of continuous variables between the two groups was performed using the unpaired t-test or the Mann-Whitney test based on the results of the Shapiro-Wilk test for normality. A comparison of categorical variables was performed using the χ2 test or Fisher’s exact test. Univariate and multivariate binary logistic regression and multinomial logistic regression analyses were conducted to investigate the relationship between sedation anesthesia with IV propofol and prostate cancer detection and complications rates. All statistical analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC, USA) and P values < 0.05 were considered statistically significant.
Ethics statement
The Institutional Review Board of The Catholic University of Korea, St. Vincent’s Hospital approved the study protocol (VC20RESI0046). Informed consent was submitted by all subjects when they were enrolled.
RESULTS
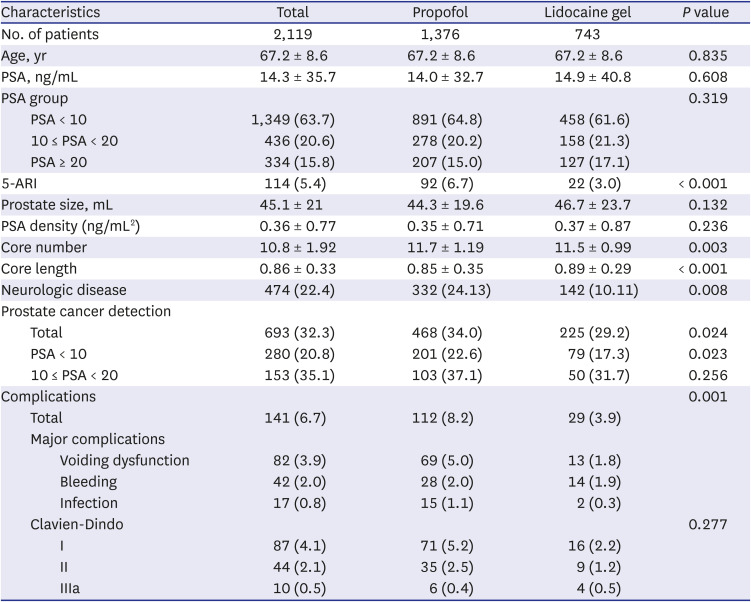
Comparison of baseline characteristics, prostate cancer detection rates, and complications rates between patients who underwent sedation anesthesia with IV propofol infusion and patients who underwent local anesthesia with intrarectal lidocaine gel instillation was performed, and the results are described in
Table 1. Among the 2,119 patients who underwent TRUS-guided prostate biopsy, 1,376 underwent sedation anesthesia and 743 underwent local anesthesia. There were no differences in age, PSA group, prostate size, and PSA density between the two groups. Core length was significantly shorter and proportion of neurologic disease was significantly higher in the sedation group (
P < 0.001 and 0.008, respectively). The cancer detection rate of all patients in the sedation group was 34.0%, whereas it was 29.2% in the local group (
P = 0.024). When the result was stratified according to PSA groups, in patients with PSA < 10, the prostate cancer detection rate was higher in the sedation group compared to the local group (22.6% vs. 17.3%,
P = 0.023). In patients with 10 ≤ PSA < 20, the prostate cancer detection rate was higher in the sedation group compared to the local group but did not exhibit a statistical significance (37.1% vs. 31.7%,
P = 0.256). The complication rate was higher in the sedation group, especially for voiding-related complications (5.0% vs. 1.8%,
P = 0.001). There was no difference in bleeding and infectious complications. There was one case of atrial flutter in the local group and one case of hyponatremia in the sedation group, both of whom recovered with no further sequelae.
Table 1
Comparison of baseline characteristics, prostate cancer detection rate and complications rate between patients who underwent sedation anesthesia with intravenous propofol infusion and patients who underwent local anesthesia with intrarectal lidocaine gel instillation
|
Characteristics |
Total |
Propofol |
Lidocaine gel |
P value |
|
No. of patients |
2,119 |
1,376 |
743 |
|
|
Age, yr |
67.2 ± 8.6 |
67.2 ± 8.6 |
67.2 ± 8.6 |
0.835 |
|
PSA, ng/mL |
14.3 ± 35.7 |
14.0 ± 32.7 |
14.9 ± 40.8 |
0.608 |
|
PSA group |
|
|
|
0.319 |
|
PSA < 10 |
1,349 (63.7) |
891 (64.8) |
458 (61.6) |
|
10 ≤ PSA < 20 |
436 (20.6) |
278 (20.2) |
158 (21.3) |
|
PSA ≥ 20 |
334 (15.8) |
207 (15.0) |
127 (17.1) |
|
5-ARI |
114 (5.4) |
92 (6.7) |
22 (3.0) |
< 0.001 |
|
Prostate size, mL |
45.1 ± 21 |
44.3 ± 19.6 |
46.7 ± 23.7 |
0.132 |
|
PSA density (ng/mL2) |
0.36 ± 0.77 |
0.35 ± 0.71 |
0.37 ± 0.87 |
0.236 |
|
Core number |
10.8 ± 1.92 |
11.7 ± 1.19 |
11.5 ± 0.99 |
0.003 |
|
Core length |
0.86 ± 0.33 |
0.85 ± 0.35 |
0.89 ± 0.29 |
< 0.001 |
|
Neurologic disease |
474 (22.4) |
332 (24.13) |
142 (10.11) |
0.008 |
|
Prostate cancer detection |
|
|
|
|
|
Total |
693 (32.3) |
468 (34.0) |
225 (29.2) |
0.024 |
|
PSA < 10 |
280 (20.8) |
201 (22.6) |
79 (17.3) |
0.023 |
|
10 ≤ PSA < 20 |
153 (35.1) |
103 (37.1) |
50 (31.7) |
0.256 |
|
Complications |
|
|
|
0.001 |
|
Total |
141 (6.7) |
112 (8.2) |
29 (3.9) |
|
Major complications |
|
|
|
|
|
Voiding dysfunction |
82 (3.9) |
69 (5.0) |
13 (1.8) |
|
|
Bleeding |
42 (2.0) |
28 (2.0) |
14 (1.9) |
|
|
Infection |
17 (0.8) |
15 (1.1) |
2 (0.3) |
|
Clavien-Dindo |
|
|
|
0.277 |
|
|
I |
87 (4.1) |
71 (5.2) |
16 (2.2) |
|
|
II |
44 (2.1) |
35 (2.5) |
9 (1.2) |
|
|
IIIa |
10 (0.5) |
6 (0.4) |
4 (0.5) |
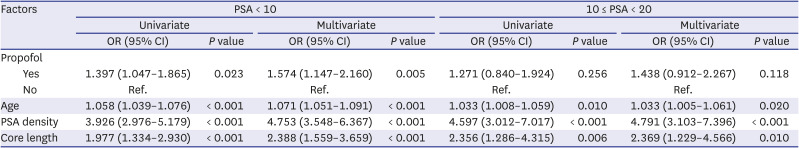
Univariate and multivariate analyses of factors related to cancer detection rate were performed and are described in
Table 2. Only patients with PSA < 10 and 10 ≤ PSA < 20 were analyzed. In patients with PSA < 10, multivariate analysis showed that age, PSA density, core length, and sedation anesthesia with IV propofol were significant factors for prostate cancer detection (odds ratio [OR], 1.574; 95% confidence interval [CI], 1.147–2.160;
P = 0.005). In patients with 10 ≤ PSA < 20, multivariate analysis showed that age, PSA density, and core length were significant factors for cancer detection, but sedation anesthesia with IV propofol was not.
Table 2
Univariate and multivariate analysis of factors related to cancer detection in patients with PSA < 10 and 10 ≤ PSA < 20
|
Factors |
PSA < 10 |
10 ≤ PSA < 20 |
|
Univariate |
Multivariate |
Univariate |
Multivariate |
|
OR (95% CI) |
P value |
OR (95% CI) |
P value |
OR (95% CI) |
P value |
OR (95% CI) |
P value |
|
Propofol |
|
|
|
|
|
|
|
|
|
Yes |
1.397 (1.047–1.865) |
0.023 |
1.574 (1.147–2.160) |
0.005 |
1.271 (0.840–1.924) |
0.256 |
1.438 (0.912–2.267) |
0.118 |
|
No |
Ref. |
|
Ref. |
|
Ref. |
|
Ref. |
|
|
Age |
1.058 (1.039–1.076) |
< 0.001 |
1.071 (1.051–1.091) |
< 0.001 |
1.033 (1.008–1.059) |
0.010 |
1.033 (1.005–1.061) |
0.020 |
|
PSA density |
3.926 (2.976–5.179) |
< 0.001 |
4.753 (3.548–6.367) |
< 0.001 |
4.597 (3.012–7.017) |
< 0.001 |
4.791 (3.103–7.396) |
< 0.001 |
|
Core length |
1.977 (1.334–2.930) |
< 0.001 |
2.388 (1.559–3.659) |
< 0.001 |
2.356 (1.286–4.315) |
0.006 |
2.369 (1.229–4.566) |
0.010 |
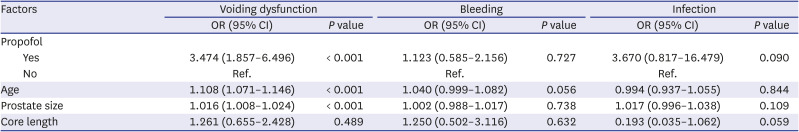
Multivariate analysis of factors related to complications after prostate biopsy was performed and is described in
Table 3. Analysis was performed separately for each complication (voiding dysfunction, bleeding, and infection), and all patients were included. For voiding dysfunction, age and prostate size were significant factors as expected, as was sedation anesthesia with IV propofol (OR, 3.474; 95% CI, 1.857–6.496;
P < 0.001). There was no significant factor for bleeding and infectious complications.
Table 3
Multivariate analysis of factors related to complications after prostate biopsy
|
Factors |
Voiding dysfunction |
Bleeding |
Infection |
|
OR (95% CI) |
P value |
OR (95% CI) |
P value |
OR (95% CI) |
P value |
|
Propofol |
|
|
|
|
|
|
|
Yes |
3.474 (1.857–6.496) |
< 0.001 |
1.123 (0.585–2.156) |
0.727 |
3.670 (0.817–16.479) |
0.090 |
|
No |
Ref. |
|
Ref. |
|
Ref. |
|
|
Age |
1.108 (1.071–1.146) |
< 0.001 |
1.040 (0.999–1.082) |
0.056 |
0.994 (0.937–1.055) |
0.844 |
|
Prostate size |
1.016 (1.008–1.024) |
< 0.001 |
1.002 (0.988–1.017) |
0.738 |
1.017 (0.996–1.038) |
0.109 |
|
Core length |
1.261 (0.655–2.428) |
0.489 |
1.250 (0.502–3.116) |
0.632 |
0.193 (0.035–1.062) |
0.059 |
DISCUSSION
Although anesthesia has been widely used for TRUS-guided prostate biopsy, study of its efficacy has mainly focused on reduction of pain or discomfort, patient satisfaction, and willingness to undergo re-biopsy. Intrarectal lidocaine gel instillation or periprostatic lidocaine injection is the most widely used method of anesthesia with efficacy in alleviating pain and discomfort during TRUS-guided prostate biopsy.
1415 However, studies exist that question this efficacy in pain reduction and variability of reported success rates,
15161718 as well as its ineffectiveness in alleviating patient anxiety associated with the procedure, leading to reduced patient tolerance and acceptability for future repeat biopsies.
11 IV anesthesia on the other hand, was found to have the added benefit of significantly more patients willing to undergo re-biopsy, presumably due to its anxiolytic and amnestic effects.
1119 This was the main reason why our institution switched from performing TRUS-guided prostate biopsy with intrarectal lidocaine gel instillation to performing it with IV sedation, not with periprostatic lidocaine injection which lacks anxiolytic and amnestic properties. Our institution has recently conducted a prospective observational study that showed superior reduction in pain and anxiety as well as enhanced re-biopsy compliance with sedation anesthesia compared to local anesthesia with lidocaine.
20
Compared to the number of studies on association of sedation anesthesia and patient pain during TRUS-guided prostate biopsy, few studies have examined its association with clinical outcomes such as cancer detection and complication rates. A prospective randomized controlled study with two groups (group 1 receiving sedation anesthesia with IV propofol and remifentanil and group 2 receiving intrarectal lidocaine gel, each with 50 patients) performed by Kang et al.,
8 reported no difference in complications between the two groups. However, their study numbers were relatively small, voiding dysfunction was not included in their records, and cancer detection rates were not reported. The prospective study conducted by our institution assessed the cancer detection and complication rates but did not find significant differences.
20 However, the numbers were also relatively small, and cancer detection rates were not analyzed by different PSA level. Temiz et al.
21 compared cancer detection rate with different local anesthesia type for TRUS-guided prostate biopsy and found out that periprostatic nerve block was superior to intrarectal lidocaine gel anesthesia. Again, their numbers were relatively small and cancer detection rates were not analyzed by different PSA level.
Our institution had been performing TRUS-guided prostate biopsy with local anesthesia with intrarectal lidocaine gel instillation until September 2013, from which a complete transition to sedation anesthesia with IV propofol was conducted. As such, we were able to compare various clinical parameters including cancer detection and complication rates in a large number of patients. The large sample size allowed us to compare these parameters at different PSA levels (PSA < 10, 10 ≤ PSA < 20, and 20 ≤ PSA), unlike other studies. The results showed that patients who underwent sedation anesthesia with IV propofol exhibited a higher cancer detection rate than those who underwent local anesthesia with intrarectal lidocaine gel instillation. In multivariate analysis, sedation anesthesia with IV propofol was a significant factor for higher cancer detection rate, especially in patients with PSA < 10. Another interesting result was that sedation anesthesia with IV propofol was associated with a significantly higher rate of voiding dysfunction.
The reason for this result, however, remains unclear. Our initial hypothesis was that core length would be longer with sedation anesthesia and would lead to a higher cancer detection rate, but it was not supported. We presume that less agitation and movement during the procedure may have led to the more precise targeting of hypoechoic lesions. It is reasonable to hypothesize that sedation would provide a more stable and controlled environment to perform prostate biopsy, which could result in a more precise targeting of suspicious lesions, leading to higher cancer detection rates and fewer complications such as bleeding. Temiz et al.,
21 who studied the effect of different local anesthesia on prostate cancer detection rate, also postulated that better pain control may have helped manipulating the sonographic probe for better visualization leading to improved cancer detection rate.
The association of propofol with voiding dysfunction is not well-known and studies about the topic is scarce. Animal studies have shown suppressed micturition reflex and decreased detrusor contraction with propofol usage.
2223 In addition, a retrospective comparative study reported that children who underwent voiding cystourethrography with propofol sedation were less likely to void to completion compared those without sedation.
24 These studies suggest that propofol usage may lead to voiding dysfunction, but they are not enough to be conclusive. Alpha blockers could be used to prevent voiding complications associated with sedation anesthesia after TRUS-guided prostate biopsy. Previous studies have shown that pre-biopsy tamsulosin can decrease voiding impairment after prostate biopsy.
25 Further studies are needed with regard to these issues.
The main emphasis of the current study is not that sedative anesthesia is the best method of anaesthesia for performing TRUS-guided prostate biopsy, but rather that cancer detection rate could be an important factor to consider along with degree of analgesia, simplicity and invasiveness of procedure, cost, etc., when selecting for the optimal anesthesia for TRUS-guided prostate biopsy. Although sedation anesthesia may require more resources and cost than local anesthesia, it can still be performed as an office procedure, and is better in terms of pain control and willing to undergo second procedure. If there is a definite advantage in cancer detection rate with the use of sedation anaesthesia for TRUS-guided prostate biopsy, it can be an attractive alternative to local anaesthesia.
Our choice of propofol for sedation anesthesia should be briefly mentioned. An ideal agent for procedural sedation is one with rapid onset and recovery, effective sedation, and acceptable safety profiles.
2627 Most of the agents used for procedural sedation have the common property of dose-dependent respiratory and cardiovascular depression. Midazolam, a commonly used benzodiazepine with anxiolytic, sedative, hypnotic, and amnestic effects, has a relatively lower risk of cardiopulmonary complications because of the existence of an antidote but requires relatively longer times of onset and recovery. On the other hand, propofol has faster onset and faster recovery properties than midazolam, but the risk of respiratory and cardiovascular depression is higher. Dose adjustments are necessary with age, comorbidities, and especially with narcotics use, which is very common for propofol usage due to lack of analgesic effects. Also, it does not have an antidote like midazolam. Appropriate equipment and personnel are necessary for monitoring patients. Our institution uses propofol not only because of its fast action, but also because an anesthesiologist is always present to monitor the entire sedation procedure. Since we started using propofol in 2013, we have not experienced any serious complications related to sedation anesthesia. If an institution should choose to adopt sedation anesthesia, the choice of sedative agent should be made depending on available resources and personnel.
Several limitations of this study should be addressed. First, this was a retrospective study with the inherent possibility of selection bias and lacked information that may have affected the results such as whether patients were using alpha-blockers before the biopsy procedure. Despite this limitation, ability to analyze a large sample is an advantage of a retrospective study and is useful for examining cancer detection and complication rates, which would be difficult in a prospective study with a limited sample size. Also, because the two method was performed in different period of time (biopsies done with IV anesthesia in the later period), the more recent biopsies may have yielded better cancer detection rates. Second, cost analysis was not performed. Since the study population was involved over a 10-year period, several changes of coverage plan from the national insurance system made it difficult to perform cost comparisons. One of the most significant changes was added coverage for prostate sonogram, which decreased the amount patients pay to be similar to that of sedation anesthesia. A previous study conducted in South Korea showed an approximately 22% increased overall cost for TRUS-guided prostate biopsy with sedation anesthesia,
8 but these numbers may vary by region. Third, a comparison with periprostatic lidocaine injection, the most common anesthesia method for TRUS-guided prostate biopsy according to the literature, was not done. We did not perform this method and the practice is relatively less preferred in South Korea compared to other countries. Lastly, clinical significance of the prostate cancer was not studied.
In conclusion, sedation anesthesia with IV propofol during TRUS-guided prostate biopsy was associated with a higher cancer detection rate than was local anesthesia with intrarectal lidocaine gel instillation, especially in patients with PSA < 10. However, it also was associated with a significantly higher rate of voiding dysfunction. Cancer detection rate could be an important factor to consider when selecting for the optimal anesthesia for TRUS-guided prostate biopsy.







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download