INTRODUCTION
In patients undergoing percutaneous coronary intervention (PCI) for acute coronary syndrome (ACS), identifying and managing patients with high bleeding risk (HBR) are of major concern since prolonged 12-month dual antiplatelet therapy (DAPT) with aspirin and P2Y
12 receptor inhibitor is recommended.
1)2)3) Recently, 2 definitions for HBR, the Academic Research Consortium for HBR (ARC-HBR) criteria and the Predicting Bleeding Complications in Patients Undergoing Stent Implantation and Subsequent DAPT (PRECISE-DAPT) score, have been proposed to provide consistency in defining HBR and aid clinical decision-making for patients undergoing PCI.
3)4) Studies have shown that HBR by these 2 definitions is associated with increased risk of both bleeding and ischemic events.
5)6)7) Furthermore, newer antiplatelet agents, such as ticagrelor or prasugrel, with better speed, potency, and consistent effect have become available, and ticagrelor monotherapy after short-term DAPT may minimize bleeding risk without increasing ischemic risk.
8)9)10) The Ticagrelor Monotherapy After 3 Months in the Patients Treated With New Generation Sirolimus-eluting Stent for Acute Coronary Syndrome (TICO) trial demonstrated that ticagrelor monotherapy after 3-month DAPT was associated with a lower incidence of a composite outcome of major bleeding and adverse cardiac and cerebrovascular events at 1 year than currently recommended ticagrelor-based 12-month DAPT in ACS patients treated with drug eluting stents (DESs).
11)
Thus, the aims of the present post-hoc analysis of the TICO trial were: 1) to apply HBR by ARC-HBR criteria and PRECISE-DAPT score for clinical outcomes; and 2) to investigate the impact of ticagrelor monotherapy after 3-month DAPT in HBR compared to non-HBR patients.
METHODS
Ethical statement
The trial was approved by the Institutional Review Board of Yonsei University College of Medicine (approval No. 1-2014-0066) and at each participating center and followed the ethical principles of the Declaration of Helsinki 2013. All patients gave written informed consent prior to participation in the trial.
Study subjects and design
The study design and rationale for the TICO trial have been described in detail previously.
11)12) Briefly, the multicenter, prospective, open-label, randomized superiority trial evaluated ticagrelor monotherapy followed by 3-month DAPT versus ticagrelor-based 12-month DAPT in 3,056 patients treated with DESs for ACS (unstable angina, non-ST-elevation myocardial infarction [MI], or ST-elevation MI) in South Korea. Consenting patients were randomized in a 1:1 ratio to either therapy and observed for up to 12 months.
In this post-hoc analysis, patients were classified into ticagrelor monotherapy after 3-month DAPT and ticagrelor-based 12-month DAPT on an intention-to-treat basis for primary analysis, and those who encountered adverse events during the first 3 months after PCI were excluded (n=76). Consequently, a total of 2980 TICO patients were included in this study. Of those, 1,489 received ticagrelor monotherapy after 3-month DAPT, and 1,491 received ticagrelor-based 12-month DAPT. A per-protocol analysis was also performed to evaluate the robustness of the results obtained by intention-to-treat analysis.
Study proceedings
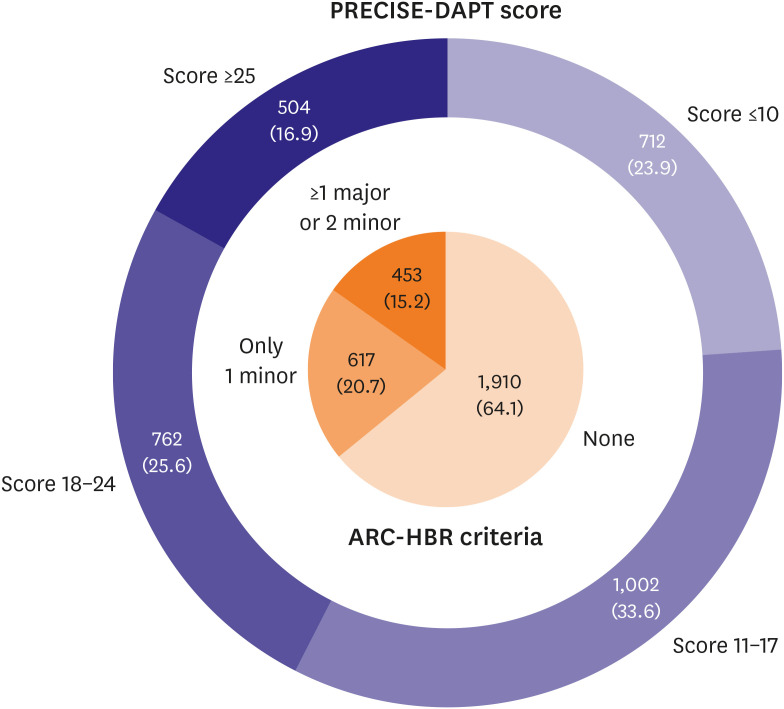
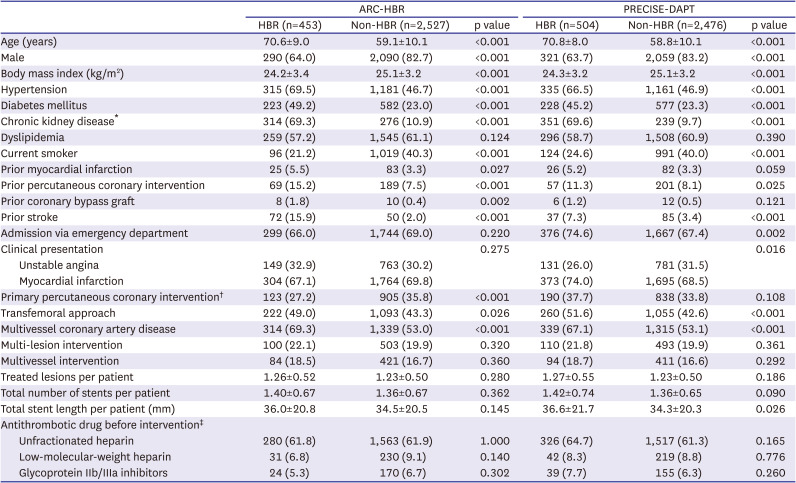
Study patients were classified by HBR using 2 different approaches: 1) by meeting ARC-HBR criteria or 2) by PRECISE-DAPT score for HBR.
3)4) Patients were considered to be at HBR if at least 1 major or 2 minor ARC-HBR criteria were met.
3) Major criteria available for analysis were severe or end-stage chronic kidney disease (estimated glomerular filtration rate <30 mL/min/1.73 m
2 or dialysis), hemoglobin <11 g/dL, previous spontaneous bleeding requiring hospitalization or transfusion within the past 6 months, and active malignancy (excluding nonmelanoma skin cancer) within the past 12 months. Minor criteria available were age ≥75 years, moderate chronic kidney disease (estimated glomerular filtration rate ≥30 and <60 mL/min/1.73 m
2), hemoglobin ≥11 and <13 g/dL for male and ≥11 and <12 g/dL for female, use of non-steroidal anti-inflammatory drugs or steroids, and any ischemic stroke beyond the past 12 months. PRECISE-DAPT scores were assessed using an online calculator (
http://www.precisedaptscore.com) with 5 variables (age, creatine clearance, hemoglobin, white blood cell count, and previous spontaneous bleeding).
4) Scores were categorized into 4 groups of bleeding risks (very low risk: score ≤10; low risk: score 11–17; moderate risk: score 18–24; and high risk: score ≥25).
Study endpoints
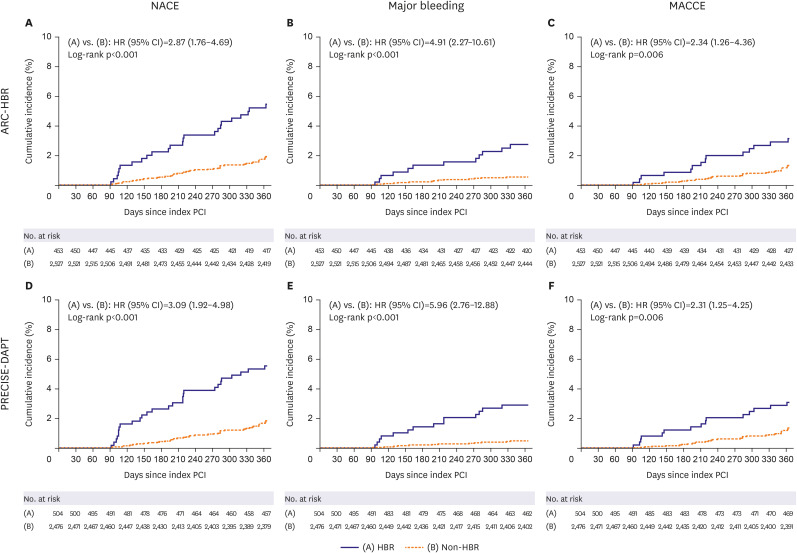
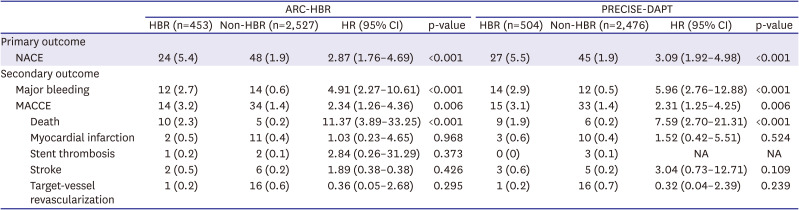
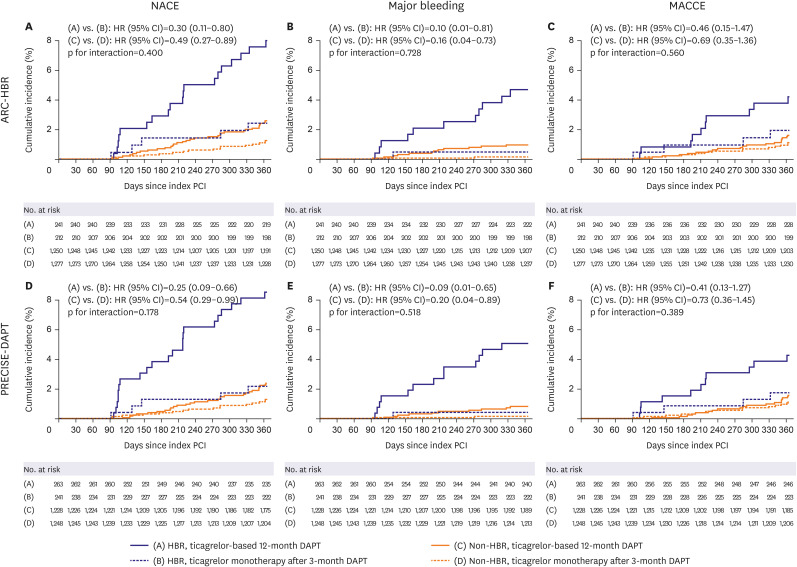
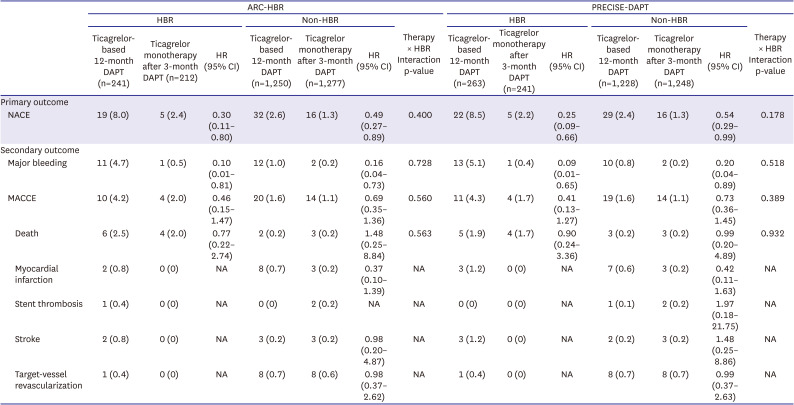
The primary outcome was a net adverse clinical event (NACE), defined as a composite of major bleeding and major adverse cardiac and cerebrovascular events (MACCEs) from 3–12 months after index PCI. MACCE included all-cause death, MI, stent thrombosis, stroke, and target-vessel revascularization. Secondary outcomes included major bleeding and MACCEs.
12)
Major bleeding was defined in accordance with the thrombolysis in MI criteria: intracranial bleeding, hemorrhage associated with a ≥5 g/dL decrease in hemoglobin, or fatal bleeding that resulted in death within 7 days.
13) MI after discharge from the hospital was defined as clinical symptoms, electrocardiography changes, or abnormal imaging findings, combined with a creatine kinase MB fraction above the upper normal limits or a troponin T or troponin I level >99th percentile of the upper normal limit.
14) Stent thrombosis was defined as definite or probable stent thrombosis according to the Academic Research Consortium.
15) Stroke was defined as an acute cerebrovascular event that resulted in death or a neurological deficit lasting >24 hours or as an acute infarction demonstrated by imaging studies.
16) Target-vessel revascularization was defined as a repeat PCI or bypass surgery of the target-vessel with either: 1) ischemia symptoms or a positive stress test and angiographic diameter stenosis >50%; or 2) angiographic diameter stenosis >70% without ischemia symptoms or a positive stress test.
12)17) Routine follow-up of angiography was not recommended in the trial. Adverse events, including bleeding and ischemic events, were categorized by an independent clinical event committee blinded to the treatment assignments and primary results of the trial.
12)
Statistical analysis
Continuous variables were reported as mean ± SD and compared using Student’s t-tests or Mann-Whitney tests. Categorical variables were reported as number (percentage) and compared using χ2 tests or Fisher’s exact tests.
Event rates were estimated using Kaplan-Meier survival analysis and compared using log-rank tests. Hazard ratios (HRs) with 95% confidence intervals (CIs) were computed using Cox regression analysis. Cox regression analysis with tests for interaction were used to assess for differential therapy effects by HBR. All tests were 2-sided, and p<0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS, version 25.0 (IBM Corporation, Chicago, IL, USA) and R 3.5.3 software (R foundations for Statistical Computing, Vienna, Austria).
DISCUSSION
The main findings of this post-hoc analysis were as follows: 1) for patients presenting with ACS and treated with DESs, HBR was associated with higher rate of primary outcome, not only including bleeding but also ischemic events; 2) ticagrelor monotherapy after 3-month DAPT was associated with lower rate of primary outcome and major bleeding than ticagrelor-based 12-month DAPT regardless of HBR, with no significant interaction between therapy and HBR; and 3) These findings were consistent regardless of HBR definition by ARC-HBR criteria or by PRECISE-DAPT score.
Current guidelines recommend 12-month DAPT including novel antiplatelet agents with higher potency in patients treated with DESs for ACS.
1)2)8)9) However, major concerns toward increased risk of bleeding by prolonged DAPT have been raised and shortened duration of DAPT may be considered in patients with HBR.
18) Therefore, it is of great importance to identify patients with HBR. The TWILIGHT trial included patients with not only high ischemic risk but also HBR, and it showed that ticagrelor monotherapy followed by 3-month DAPT after PCI was associated with lower bleeding events than ongoing ticagrelor plus aspirin, without an increased risk for ischemic events.
10) The TICO trial exclusively focused on patients with ACS and demonstrated that ticagrelor monotherapy after 3-month DAPT was associated with a lower incidence of NACE than currently recommended ticagrelor-based 12-month DAPT.
11) In this post-hoc analysis, we classified the TICO subjects into HBR and non-HBR patients by 2 different definitions of HBR that were proposed recently: the ARC-HBR criteria and the PRECISE-DAPT score.
3)4) The ARC-HBR criteria, which consists of 20 clinical criteria as major and minor by consensus and supported by published evidence, and the PRECISE-DAPT score, which consists of 5 variables developed from a large pooled dataset of recent randomized clinical trials, were excellent in identifying those with HBR after PCI and associated with higher risk of bleeding as well as ischemic events when validated to Bern PCI registry.
3)4)5)6)7) In our study, the rate of NACE, including major bleeding and MACCE, was significantly higher in HBR versus non-HBR patients, regardless of the HBR definition which was consistent to those of previous studies.
Several studies have investigated the association between HBR and the duration of DAPT. Costa et al. reported that compared to a short DAPT duration (3–6 months), a long DAPT duration (12–24 months) increased bleeding in patients with HBR but not in those with non-HBR (p for interaction=0.007).
4) According to the secondary analysis, 20.8% of patients underwent complex PCI, which is associated with higher ischemic risk and can be alleviated by a long DAPT duration.
19) However, the benefit of a long DAPT duration for reducing ischemic events was present only in patients with non-HBR for both complex (absolute risk difference: −3.86%, p=0.05) and noncomplex PCI (absolute risk difference: −1.14%, p=0.04). The benefit was not present in HBR patients for both complex (absolute risk difference: +1.30%, p=0.76) and noncomplex PCI (absolute risk difference: +1.45%, p=0.39), which indicated that bleeding, more than ischemic risk, should inform on making decision for DAPT duration after PCI.
19) Different from previous studies, only ultrathin bioresorbable polymer sirolimus-eluting stents (Orsiro; Biotronik AG, Bülach, Switzerland) were used in the TICO trial, which have shown superior clinical outcomes over other DESs, probably due to reduced thrombus formation, inflammation, and neointimal proliferation.
11)12)20)21) Likewise, as for DAPT strategies utilizing P2Y
12 receptor inhibitor, ticagrelor was used, which showed superior clinical outcomes over clopidogrel in reducing ischemic events.
8) Therefore, long DAPT duration for the purpose of preventing ischemic events is less likely needed compared to other DESs and P2Y
12 receptor inhibitors, which raises the issue of bleeding risk to be considered as a priority over ischemic risk. Furthermore, although the definition of short and long duration of DAPT was heterogeneous (3–6 months vs. 12–24 months) with aspirin continuation after DAPT in previous studies, the definition was strictly confined to 3-month DAPT with P2Y
12 receptor inhibitor continuation versus 12-month DAPT in the TICO trial.
4)11)19) These factors may have contributed to the findings of this study, which showed the beneficial effect of ticagrelor monotherapy after short DAPT duration in both HBR and non-HBR patients. However, the benefit of ticagrelor monotherapy after 3-month DAPT tended to be more prominent in HBR patients which is in line with the secondary analysis of the TWILIGHT trial regarding HBR.
22)
This study has several limitations. First, the present study was not prespecified in the TICO trial protocol; however, the definition of HBR by ARC-HBR criteria or PRECISE-DAPT score was not developed at the time of trial design. Therefore, our findings need to be interpreted only as hypothesis-generating and require further prospective randomized trials. Second, the number of HBR patients is relatively small compared with non-HBR patients. Not all criteria for ARC-HBR were available for analysis, and while some of those were not routinely collected in the TICO trial, others were in fact exclusion criteria for the trial since the patients with an extremely high risk of bleeding who may be contraindicated for antiplatelet agents were excluded as in other trials regarding antiplatelet therapy strategies. Nevertheless, statistical significance for the benefit of ticagrelor monotherapy after 3-month DAPT toward primary outcome and major bleeding was found even in relatively small number of HBR patients. Third, the patients who encountered adverse events during the first 3 months after PCI were excluded in primary analysis, since the therapy during the first 3 months was the same in both therapy strategies. However, key findings of this post-hoc analysis (interactions between therapy and HBR for clinical outcomes) were also confirmed without excluding the patients with adverse events within 3 months. Fourth, although the superiority assumption was met, the lower than expected event rate of the primary outcome was noted in the TICO trial, which was consistent in this post-hoc analysis.
In conclusions, in patients with ACS treated with DESs, ticagrelor monotherapy after 3-month DAPT was associated with a lower incidence of NACE and major bleeding than currently recommended ticagrelor-based 12-month DAPT regardless of HBR, and the magnitude of therapy effect did not differ between HBR and non-HBR.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download