In patients with acute coronary syndrome (ACS) treated with percutaneous coronary intervention (PCI), dual antiplatelet therapy (DAPT) is an essential strategy of their management. According to the current guidelines, it is generally recommended to commence DAPT with potent P2Y12 inhibitor (ticagrelor or prasugrel) at the least for 12 months, except in patients with high- or very high bleeding risk (HBR).1)
Among ACS patients, these potent P2Y12 inhibitors are associated with a reduction in ischemic events as well as an increase in bleeding events. Therefore, finding an optimal balance between the risk and benefit of DAPT treatment (i.e., potency and duration) has been one of the most challenging tasks ahead.2) With the recent advances in stent platforms, optimized implant techniques and standardized cardiovascular (CV) medication, in addition to increasing awareness of HBR phenotype and its association with serious bleeding and CV outcomes, the clinical focus on the DAPT strategy has shifted toward reducing clinically significant bleeding events.
In specific clinical scenarios of ACS patients, DAPT duration can be shortened (<12 months: short-term DAPT, followed by P2Y12 inhibitor or aspirin monotherapy) and extended (>12 months), and the regimen of P2Y12 inhibitors can be modified (switching, de-escalation or escalation) within 12 months.1) These decisions may depend on clinical judgement driven by the patient’s weighing between ischemic and bleeding risk, the occurrence of adverse events, co-morbidities, and the availability of the switching drug or regimen. A recent meta-analysis (5 randomized clinical trials [RCTs], n=10,779) showed that DAPT de-escalation (when: from in-hospital to 1 month post-PCI) vs. standard DAPT with full-dose potent P2Y12 inhibitor was associated with a significant reduction in Bleeding Academic Research Consortium (BARC) ≥type 2 bleeding (hazard ratio [HR], 0.57; 95% confidence interval [CI], 0.42–0.78) as well as major adverse cardiac events, represented by the composite of CV mortality, myocardial infarction (MI), stent thrombosis, and stroke (HR, 0.77; 95% CI, 0.62–0.96) in ACS patients who underwent PCI.3) Although the consistency was noted across various de-escalation strategies, it cannot guarantee that this strategy can be applied globally because of its limited power and sample size.3)
In this issue of the Korean Circulation Journal, Ki et al.4) presented a pre-specified subgroup analysis of the HOST-REDUCE-POLYTECH-ACS trial (n=2,338) to assess the efficacy and safety of the de-escalation strategy (10-mg prasugrel during 1 month → 5-mg prasugrel from 1 month to 12 months) between Korean patients presented with ST-segment elevation MI (STEMI) and non-ST-segment elevation ACS, compared with the full-dose prasugrel therapy (10-mg prasugrel during 12 months). The primary findings were that unguided DAPT de-escalation strategy in those with STEMI was not associated with a statistically significant reduction of bleeding events (≥BARC type 2 bleeding, 2.3% vs. 3.9%; HR, 0.59; 95% CI, 0.17–2.08; p=0.411) and tended to have increased risk of ischemic events (2.3% vs. 1.3%; HR, 1.78; 95% CI, 0.33–9.70; p=0.507). However, these East Asian patients still showed that this de-escalation strategy from 1 month had the similar or relatively better net clinical benefit (4.6% vs. 5.2%) compared with the standard strategy in the STEMI cohort. This analysis has several limitations including specific characteristics of enrolled population (e.g., East Asian patients excluding old age and/or low body weight), and only 14% of STEMI patients underpowered to estimate clinical differences. The results may suggest that early uniform application of unguided de-escalation strategy in patients with high thrombotic condition (whom: STEMI, hypercoagulable state, complex PCI) requires a careful approach.
For ACS patients with a high ischemic and an acceptable bleeding risk, Western consensus recommends to choose extended DAPT or adding low-dose rivaroxaban to aspirin after standard DAPT (Figure 1).5) HBR phenotype may be one of the best candidates for adapting the de-escalation DAPT strategy. A major challenging issue is a case with concurrent HBR and high ischemic risk. Most of Western evidences support that in these patients HBR rather than ischemic risk should guide decision-making regarding treatment strategy.
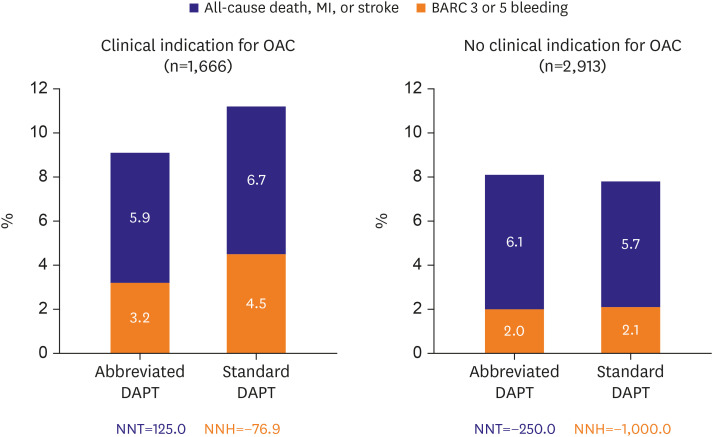
However, the results from recent RCTs gave us a lot of clinical concerns regarding uniform de-escalation strategy. The Management of High Bleeding Risk Patients Post Bioresorbable Polymer Coated Stent Implantation With an Abbreviated Versus Standard DAPT Regimen (MASTER-DAPT) trial including patients with HBR criteria (n=4,579) compared clinical benefit of abbreviated vs. conventional antiplatelet therapy strategy following 1-month DAPT.6) The antiplatelet monotherapy—mostly clopidogrel—after 1-month DAPT increased the risk of thrombotic events (especially, 2.2-fold occurrence of stent thrombosis) without difference in major bleeding compared to standard DAPT treatment in patients without clinical indication for anticoagulation (Figure 2). Another Short and Optimal Duration of Dual Antiplatelet Therapy After Everolimus-Eluting Cobalt-Chromium Stent-2 in Patient with Acute Coronary Syndrome (STOP DAPT2-ACS) trial7) enrolling Japanese ACS patients treated with less potent P2Y12 inhibitor (i.e., clopidogrel or 3.75-mg prasugrel) (n=4,136) also showed that short DAPT strategy (P2Y12 inhibitor monotherapy following 1-month DAPT) was associated with the increased risk of MI (1.59% vs. 0.85%) and stent thrombosis (0.45% vs. 0.20%), albeit at the benefit of a lower incidence of BARC 3 or 5 bleeding (0.54% vs. 1.31%). Contrary to de-escalation of P2Y12 inhibitor,3) early (≤1 month) aspirin discontinuation with clopidogrel monotherapy may increase the rate of ischemic events even in patients with low thrombotic risk (e.g., East Asian patients).8)
Instead of unguided de-escalation strategy, guided de-escalation (how: platelet function test,9) genotyping and risk score10)) can be a promising way to have a biological and clinical rationale. The robustness of the clinical evidences still does not support the routine use of these guidance tools. The level of platelet reactivity can be changed dynamically in the early period when having a strong association with clinical events.9) The prognostic implications of platelet function and genotyping on occurrence of ischemic events in East Asian patients would be lower than that in Western population.8) To date, there have been no RCTs comparing a risk score-based approach with the standard-of-care approach.10) In addition, required level of platelet inhibition to match net clinical benefit may differ according to clinical, anatomical, procedural, and laboratory aspects.
Deciding for when, whom and how to shorten vs. extend and de-escalate vs. escalate antiplatelet therapy in ACS patients must be complex. DAPT de-escalation strategy may be a piece of way to improve patients’ clinical outcomes. The precision medicine of antithrombotic therapy should be based on an individualized duration and potency guided by systematic risk stratification.5) There are multiple risk-stratifying methods (e.g., platelet function test, genotyping and risk score) to guide clinical decision-making, all with their own advantages and drawbacks. Future research should indicate how to best balance patients’ bleeding and ischemic risk, and subsequently suggest them with best patient-tailored therapy.
Notes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest: Dr. Jeong has received honoraria for lectures from AstraZeneca, Daiichi Sankyo, Sanofi-Aventis, Han-mi Pharmaceuticals and Yuhan Pharmaceuticals; and research grants or support from Yuhan Pharmaceuticals, Han-mi Pharmaceuticals, Sam-jin Pharmaceuticals, Biotronik, and U&I Corporation. The other author reports no conflicts of interest.
Data Sharing Statement: The data generated in this study is available from the corresponding authors upon reasonable request.
References
1. Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021; 42:1289–1367. PMID: 32860058.
2. Kupka D, Sibbing D. De-escalation of P2Y12 receptor inhibitor therapy after acute coronary syndromes in patients undergoing percutaneous coronary intervention. Korean Circ J. 2018; 48:863–872. PMID: 30238704.

3. Tavenier AH, Mehran R, Chiarito M, et al. Guided and unguided de-escalation from potent P2Y12 inhibitors among patients with ACS: a meta-analysis. Eur Heart J Cardiovasc Pharmacother. 2021; pvab068. PMID: 34459481.

4. Ki YJ, Lee BK, Park KW, et al. Prasugrel-based de-escalation of dual antiplatelet therapy after percutaneous coronary intervention in patients with STEMI. Korean Circ J. 2022; 52:304–319. PMID: 35129316.

5. van der Sangen NMR, Rozemeijer R, Chan Pin Yin DRPP, et al. Patient-tailored antithrombotic therapy following percutaneous coronary intervention. Eur Heart J. 2021; 42:1038–1046. PMID: 33515031.

6. Smits PC, Frigoli E, Tijssen J, et al. Abbreviated antiplatelet therapy in patients at high bleeding risk with or without oral anticoagulant therapy after coronary stenting: an open-label, randomized, controlled trial. Circulation. 2021; 144:1196–1211. PMID: 34455849.

7. Watanabe H. STOPDAPT-2 ACS: one-month dual antiplatelet therapy followed by clopidogrel monotherapy in acute coronary syndrome. In : ESC Congress; 2021 Aug 27-30.
8. Kim HK, Tantry US, Park HW, et al. Ethnic difference of thrombogenicity in patients with cardiovascular disease: a pandora box to explain prognostic differences. Korean Circ J. 2021; 51:202–221. PMID: 33655720.

9. Jeong YH, Oh JH, Yoon HJ, et al. Pharmacodynamic profile and prevalence of bleeding episode in East Asian patients with acute coronary syndromes treated with prasugrel standard-dose versus de-escalation strategy: a randomized A-MATCH trial. Thromb Haemost. 2021; 121:1376–1386. PMID: 33401330.

10. Lee SH, Kim HK, Jeong MH, et al. Practical guidance for P2Y12 inhibitors in acute myocardial infarction undergoing percutaneous coronary intervention. Eur Heart J Cardiovasc Pharmacother. 2021; 7:112–124. PMID: 31977008.

Figure 1
Patient-tailored antithrombotic strategies in patients with acute coronary syndrome (modified from van der Sangen et al.)5) - 7)
DAPT = dual antiplatelet therapy; FXa = factor Xa.
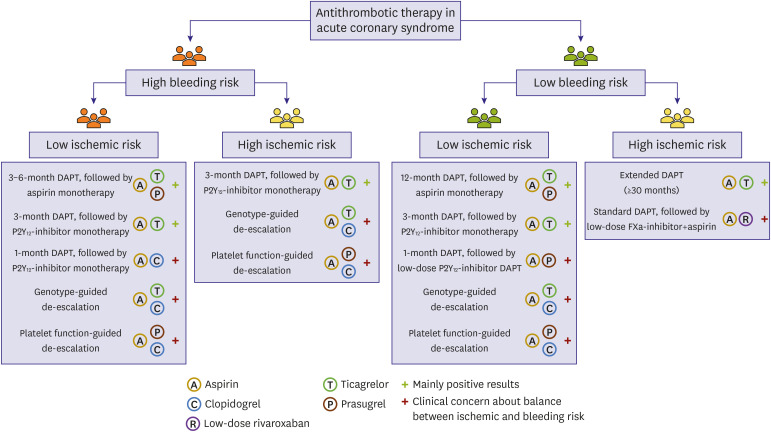
Figure 2
The MASTER-DAPT trial: net clinical benefit of abbreviated vs. stardard DAPT in patients with an OAC indication (left) and without an OAC indication (right).
BARC = Bleeding Academic Research Consortium; DAPT = dual antiplatelet therapy; MASTER-DAPT = Management of High Bleeding Risk Patients Post Bioresorbable Polymer Coated Stent Implantation With an Abbreviated Versus Standard DAPT Regimen; MI = myocardial infarction; NNH = number needed to harm; NNT = number needed to treat; OAC = oral anticoagulant.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download