2. Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020; 76:2982–3021. PMID:
33309175.
3. Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2017; 14:591–602. PMID:
28492288.

4. Luchi RJ, Snow E, Luchi JM, Nelson CL, Pircher FJ. Left ventricular function in hospitalized geriatric patients. J Am Geriatr Soc. 1982; 30:700–705. PMID:
7130576.

5. Shah KS, Xu H, Matsouaka RA, et al. Heart failure with preserved, borderline, and reduced ejection fraction: 5-year outcomes. J Am Coll Cardiol. 2017; 70:2476–2486. PMID:
29141781.
6. Conraads VM, Metra M, Kamp O, et al. Effects of the long-term administration of nebivolol on the clinical symptoms, exercise capacity, and left ventricular function of patients with diastolic dysfunction: results of the ELANDD study. Eur J Heart Fail. 2012; 14:219–225. PMID:
22147202.

7. Yamamoto K, Origasa H, Hori M. J-DHF Investigators. Effects of carvedilol on heart failure with preserved ejection fraction: the Japanese Diastolic Heart Failure Study (J-DHF). Eur J Heart Fail. 2013; 15:110–118. PMID:
22983988.

8. Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008; 359:2456–2467. PMID:
19001508.

9. Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014; 370:1383–1392. PMID:
24716680.

10. Solomon SD, McMurray JJV, Anand IS, et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019; 381:1609–1620. PMID:
31475794.

11. Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021; 385:1451–1461. PMID:
34449189.
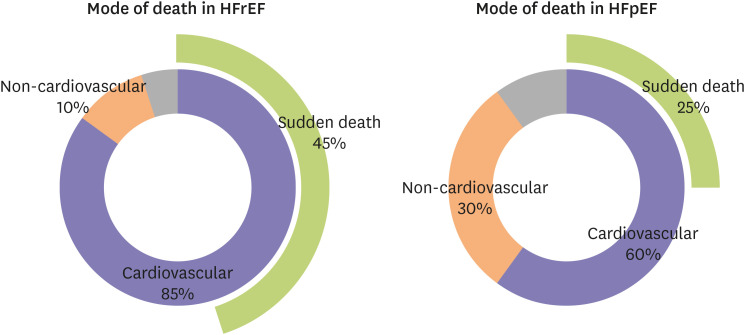
12. Vaduganathan M, Patel RB, Michel A, et al. Mode of Death in Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol. 2017; 69:556–569. PMID:
28153111.

13. Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001; 345:1473–1482. PMID:
11794197.

14. Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2018; 138:e272–e391. PMID:
29084731.
15. Risgaard B, Lynge TH, Wissenberg M, et al. Risk factors and causes of sudden noncardiac death: a nationwide cohort study in Denmark. Heart Rhythm. 2015; 12:968–974. PMID:
25614248.

16. Chugh SS, Jui J, Gunson K, et al. Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate-based review in a large U.S. community. J Am Coll Cardiol. 2004; 44:1268–1275. PMID:
15364331.

17. Tseng ZH, Olgin JE, Vittinghoff E, et al. Prospective countywide surveillance and autopsy characterization of sudden cardiac death: POST SCD study. Circulation. 2018; 137:2689–2700. PMID:
29915095.

18. Zipes DP, Wellens HJ. Sudden cardiac death. Circulation. 1998; 98:2334–2351. PMID:
9826323.

19. Stevenson WG, Stevenson LW, Middlekauff HR, Saxon LA. Sudden death prevention in patients with advanced ventricular dysfunction. Circulation. 1993; 88:2953–2961. PMID:
8252708.

20. de Vreede-Swagemakers JJ, Gorgels AP, Dubois-Arbouw WI, et al. Out-of-hospital cardiac arrest in the 1990's: a population-based study in the Maastricht area on incidence, characteristics and survival. J Am Coll Cardiol. 1997; 30:1500–1505. PMID:
9362408.

21. Basso C, Aguilera B, Banner J, et al. Guidelines for autopsy investigation of sudden cardiac death: 2017 update from the Association for European Cardiovascular Pathology. Virchows Arch. 2017; 471:691–705. PMID:
28889247.

22. Department of Health and Human Services; Centers for Disease Control and Prevention. National Center for Health Statistics. Medical examiners' and coroners' handbook on death registration and fetal death reporting. Atlanta (GA): Centers for Disease Control and Prevention;2003.
23. Murphy SL, Xu J, Kochanek KD, Curtin SC, Arias E. Deaths: final data for 2015. Natl Vital Stat Rep. 2017; 66:1–75.
24. Zile MR, Gaasch WH, Anand IS, et al. Mode of death in patients with heart failure and a preserved ejection fraction: results from the Irbesartan in Heart Failure With Preserved Ejection Fraction Study (I-Preserve) trial. Circulation. 2010; 121:1393–1405. PMID:
20231531.

25. Roberts WC, Kragel AH, Gertz SD, Roberts CS. Coronary arteries in unstable angina pectoris, acute myocardial infarction, and sudden coronary death. Am Heart J. 1994; 127:1588–1593. PMID:
8197987.

26. Doval HC, Nul DR, Grancelli HO, et al. Nonsustained ventricular tachycardia in severe heart failure. Independent marker of increased mortality due to sudden death. Circulation. 1996; 94:3198–3203. PMID:
8989129.

27. Tin LL, Beevers DG, Lip GY. Hypertension, left ventricular hypertrophy, and sudden death. Curr Cardiol Rep. 2002; 4:449–457. PMID:
12379162.

28. Hamaguchi S, Kinugawa S, Sobirin MA, et al. Mode of death in patients with heart failure and reduced vs. preserved ejection fraction: report from the registry of hospitalized heart failure patients. Circ J. 2012; 76:1662–1669. PMID:
22481105.

29. Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002; 346:877–883. PMID:
11907286.

30. Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005; 352:225–237. PMID:
15659722.

31. Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med. 1996; 335:1933–1940. PMID:
8960472.

32. Adabag S, Rector TS, Anand IS, et al. A prediction model for sudden cardiac death in patients with heart failure and preserved ejection fraction. Eur J Heart Fail. 2014; 16:1175–1182. PMID:
25302657.

33. Vaduganathan M, Claggett BL, Chatterjee NA, et al. Sudden death in heart failure with preserved ejection fraction: a competing risks analysis from the TOPCAT trial. JACC Heart Fail. 2018; 6:653–661. PMID:
29501806.

34. Cho JH, Zhang R, Aynaszyan S, et al. Ventricular tachycardia underlie sudden death in rats with heart failure and preserved ejection fraction. Circ Arrhythm Electrophysiol. 2018; 11:e006452. PMID:
30030266.

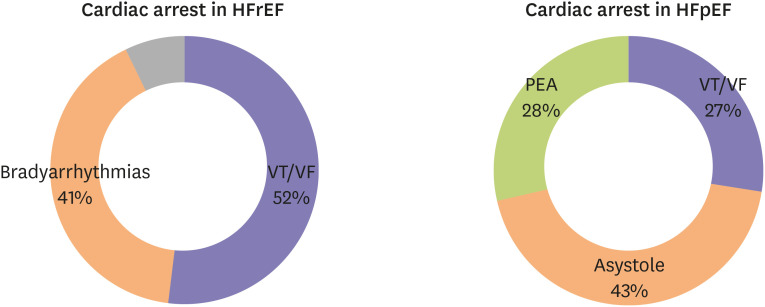
35. Woolcott OO, Reinier K, Uy-Evanado A, et al. Sudden cardiac arrest with shockable rhythm in patients with heart failure. Heart Rhythm. 2020; 17:1672–1678. PMID:
32504821.

36. Gutierrez A, Ash J, Akdemir B, et al. Nonsustained ventricular tachycardia in heart failure with preserved ejection fraction. Pacing Clin Electrophysiol. 2020; 43:1126–1131. PMID:
32809234.

37. Cho JH, Leong D, Cuk N, et al. Delayed repolarization and ventricular tachycardia in patients with heart failure and preserved ejection fraction. PLoS One. 2021; 16:e0254641. PMID:
34255806.

38. van Veldhuisen DJ, van Woerden G, Gorter TM, et al. Ventricular tachyarrhythmia detection by implantable loop recording in patients with heart failure and preserved ejection fraction: the VIP-HF study. Eur J Heart Fail. 2020; 22:1923–1929. PMID:
32683763.

39. Redfield MM. Heart failure with preserved ejection fraction. N Engl J Med. 2016; 375:1868–1877. PMID:
27959663.

40. McLenachan JM, Henderson E, Morris KI, Dargie HJ. Ventricular arrhythmias in patients with hypertensive left ventricular hypertrophy. N Engl J Med. 1987; 317:787–792. PMID:
2957590.

41. Levy D, Anderson KM, Savage DD, Balkus SA, Kannel WB, Castelli WP. Risk of ventricular arrhythmias in left ventricular hypertrophy: the Framingham Heart Study. Am J Cardiol. 1987; 60:560–565. PMID:
2957907.

42. Ghali JK, Kadakia S, Cooper RS, Liao YL. Impact of left ventricular hypertrophy on ventricular arrhythmias in the absence of coronary artery disease. J Am Coll Cardiol. 1991; 17:1277–1282. PMID:
1826691.

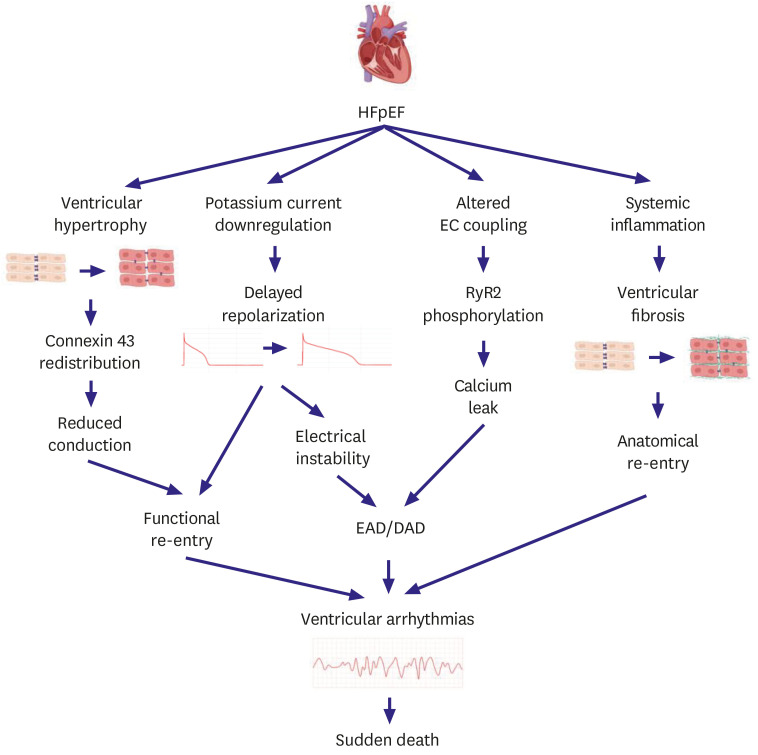
43. Kostin S, Dammer S, Hein S, Klovekorn WP, Bauer EP, Schaper J. Connexin 43 expression and distribution in compensated and decompensated cardiac hypertrophy in patients with aortic stenosis. Cardiovasc Res. 2004; 62:426–436. PMID:
15094362.

44. Akar FG, Nass RD, Hahn S, et al. Dynamic changes in conduction velocity and gap junction properties during development of pacing-induced heart failure. Am J Physiol Heart Circ Physiol. 2007; 293:H1223–H1230. PMID:
17434978.

45. Tomaselli GF, Beuckelmann DJ, Calkins HG, et al. Sudden cardiac death in heart failure. The role of abnormal repolarization. Circulation. 1994; 90:2534–2539. PMID:
7955213.

46. Tomaselli GF, Marbán E. Electrophysiological remodeling in hypertrophy and heart failure. Cardiovasc Res. 1999; 42:270–283. PMID:
10533566.

47. Wilcox JE, Rosenberg J, Vallakati A, Gheorghiade M, Shah SJ. Usefulness of electrocardiographic QT interval to predict left ventricular diastolic dysfunction. Am J Cardiol. 2011; 108:1760–1766. PMID:
21907948.

48. Cho JH, Zhang R, Kilfoil PJ, et al. Delayed repolarization underlies ventricular arrhythmias in rats with heart failure and preserved ejection fraction. Circulation. 2017; 136:2037–2050. PMID:
28974519.

49. Furukawa T, Bassett AL, Furukawa N, Kimura S, Myerburg RJ. The ionic mechanism of reperfusion-induced early afterdepolarizations in feline left ventricular hypertrophy. J Clin Invest. 1993; 91:1521–1531. PMID:
8386189.

50. Yan GX, Wu Y, Liu T, Wang J, Marinchak RA, Kowey PR. Phase 2 early afterdepolarization as a trigger of polymorphic ventricular tachycardia in acquired long-QT syndrome : direct evidence from intracellular recordings in the intact left ventricular wall. Circulation. 2001; 103:2851–2856. PMID:
11401944.

51. Weiss JN, Garfinkel A, Karagueuzian HS, Chen PS, Qu Z. Early afterdepolarizations and cardiac arrhythmias. Heart Rhythm. 2010; 7:1891–1899. PMID:
20868774.

52. Beuckelmann DJ, Näbauer M, Erdmann E. Alterations of K+ currents in isolated human ventricular myocytes from patients with terminal heart failure. Circ Res. 1993; 73:379–385. PMID:
8330380.

53. Näbauer M, Beuckelmann DJ, Erdmann E. Characteristics of transient outward current in human ventricular myocytes from patients with terminal heart failure. Circ Res. 1993; 73:386–394. PMID:
8330381.

54. Wettwer E, Amos G, Gath J, Zerkowski HR, Reidemeister JC, Ravens U. Transient outward current in human and rat ventricular myocytes. Cardiovasc Res. 1993; 27:1662–1669. PMID:
8287446.

55. Wettwer E, Amos GJ, Posival H, Ravens U. Transient outward current in human ventricular myocytes of subepicardial and subendocardial origin. Circ Res. 1994; 75:473–482. PMID:
8062421.

56. Cho JH, Kilfoil PJ, Zhang R, et al. Reverse electrical remodeling in rats with heart failure and preserved ejection fraction. JCI Insight. 2018; 3:e121123.

57. Marx SO, Reiken S, Hisamatsu Y, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000; 101:365–376. PMID:
10830164.

58. Farr MA, Basson CT. Sparking the failing heart. N Engl J Med. 2004; 351:185–187. PMID:
15247360.

59. Kilfoil PJ, Lotteau S, Zhang R, et al. Distinct features of calcium handling and β-adrenergic sensitivity in heart failure with preserved versus reduced ejection fraction. J Physiol. 2020; 598:5091–5108. PMID:
32829489.

60. Frisk M, Le C, Shen X, et al. Etiology-dependent impairment of diastolic cardiomyocyte calcium homeostasis in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2021; 77:405–419. PMID:
33509397.

61. Runte KE, Bell SP, Selby DE, et al. Relaxation and the role of calcium in isolated contracting myocardium from patients with hypertensive heart disease and heart failure with preserved ejection fraction. Circ Heart Fail. 2017; 10:e004311. PMID:
28784688.

62. Dridi H, Kushnir A, Zalk R, Yuan Q, Melville Z, Marks AR. Intracellular calcium leak in heart failure and atrial fibrillation: a unifying mechanism and therapeutic target. Nat Rev Cardiol. 2020; 17:732–747. PMID:
32555383.

63. Kass DA, Bronzwaer JG, Paulus WJ. What mechanisms underlie diastolic dysfunction in heart failure? Circ Res. 2004; 94:1533–1542. PMID:
15217918.

64. Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013; 62:263–271. PMID:
23684677.
65. Mesquita T, Lin YN, Ibrahim A. Chronic low-grade inflammation in heart failure with preserved ejection fraction. Aging Cell. 2021; 20:e13453. PMID:
34382743.

66. Paulus WJ, Zile MR. From systemic inflammation to myocardial fibrosis: the heart failure with preserved ejection fraction paradigm revisited. Circ Res. 2021; 128:1451–1467. PMID:
33983831.
67. Mishra S, Kass DA. Cellular and molecular pathobiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2021; 18:400–423. PMID:
33432192.

68. Gallet R, de Couto G, Simsolo E, et al. Cardiosphere-derived cells reverse heart failure with preserved ejection fraction (HFpEF) in rats by decreasing fibrosis and inflammation. JACC Basic Transl Sci. 2016; 1:14–28. PMID:
27104217.

69. Schiattarella GG, Altamirano F, Tong D, et al. Nitrosative stress drives heart failure with preserved ejection fraction. Nature. 2019; 568:351–356. PMID:
30971818.

70. Rockey DC, Bell PD, Hill JA. Fibrosis--a common pathway to organ injury and failure. N Engl J Med. 2015; 373:96.
71. Schelbert EB, Fridman Y, Wong TC, et al. Temporal relation between myocardial fibrosis and heart failure with preserved ejection fraction: association with baseline disease severity and subsequent outcome. JAMA Cardiol. 2017; 2:995–1006. PMID:
28768311.

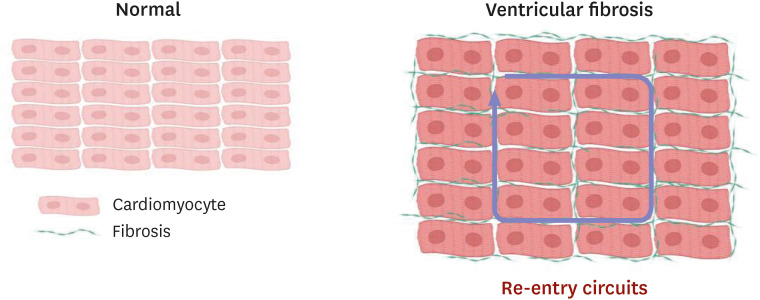
72. Ten Tusscher KH, Panfilov AV. Influence of diffuse fibrosis on wave propagation in human ventricular tissue. Europace. 2007; 9 Suppl 6:vi38–vi45. PMID:
17959692.

73. Nguyen TP, Qu Z, Weiss JN. Cardiac fibrosis and arrhythmogenesis: the road to repair is paved with perils. J Mol Cell Cardiol. 2014; 70:83–91. PMID:
24184999.






 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download