1. Cohen M, Quintner J, van Rysewyk S. Reconsidering the International Association for the Study of Pain definition of pain. Pain Rep. 2018; 3:e634.

2. Skrobik Y, Ahern S, Leblanc M, Marquis F, Awissi DK, Kavanagh BP. Protocolized intensive care unit management of analgesia, sedation, and delirium improves analgesia and subsyndromal delirium rates. Anesth Analg. 2010; 111:451–63.

3. Kim T, Kim JS, Choi EY, Chang Y, Choi WI, Hwang JJ, et al. Utilization of pain and sedation therapy on noninvasive mechanical ventilation in Korean intensive care units: a multi-center prospective observational study. Acute Crit Care. 2020; 35:255–62.

4. Balas MC, Weinhouse GL, Denehy L, Chanques G, Rochwerg B, Misak CJ, et al. Interpreting and implementing the 2018 pain, agitation/sedation, delirium, immobility, and sleep disruption clinical practice guideline. Crit Care Med. 2018; 46:1464–70.

5. Desbiens NA, Wu AW, Broste SK, Wenger NS, Connors AF Jr, Lynn J, et al. Pain and satisfaction with pain control in seriously ill hospitalized adults: findings from the SUPPORT research investigations. For the SUPPORT investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatmentm. Crit Care Med. 1996; 24:1953–61.
6. Chanques G, Jaber S, Barbotte E, Violet S, Sebbane M, Perrigault PF, et al. Impact of systematic evaluation of pain and agitation in an intensive care unit. Crit Care Med. 2006; 34:1691–9.

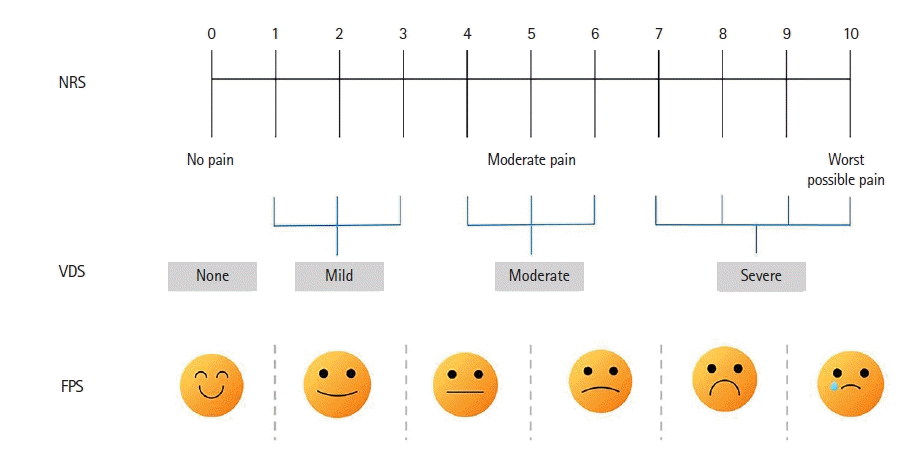
7. Chanques G, Viel E, Constantin JM, Jung B, de Lattre S, Carr J, et al. The measurement of pain in intensive care unit: comparison of 5 self-report intensity scales. Pain. 2010; 151:711–21.

8. Karahan A. Comparison of three rating scales for assessing pain intensity in an intensive care unit. Turk J Thorac Cardiovasc Surg. 2012; 20:50–5.

9. Gélinas C. The Faces Pain Thermometer: a new tool for critically ill adults. Perspect Infirm. 2007; 4:12–20.
10. Payen JF, Bru O, Bosson JL, Lagrasta AR, Novel EP, Deschaux IR, et al. Assessing pain in critically ill sedated patients by using a behavioral pain scale. Crit Care Med. 2001; 29:2258–63.

11. Gélinas C, Fillion L, Puntillo KA, Viens C, Fortier M. Validation of the critical-care pain observation tool in adult patients. Am J Crit Care. 2006; 15:420–7.

12. Puntillo KA, Neuhaus J, Arai S, Paul SM, Gropper MA, Cohen NH, et al. Challenge of assessing symptoms in seriously ill intensive care unit patients: can proxy reporters help? Crit Care Med. 2012; 40:2760–7.
13. Desbiens NA, Mueller-Rizner N. How well do surrogates assess the pain of seriously ill patients? Crit Care Med. 2000; 28:1347–52.

14. Kapoustina O, Echegaray-Benites C, Gélinas C. Fluctuations in vital signs and behavioural responses of brain surgery patients in the intensive care unit: are they valid indicators of pain? J Adv Nurs. 2014; 70:2562–76.

15. Payen JF, Chanques G, Mantz J, Hercule C, Auriant I, Leguillou JL, et al. Current practices in sedation and analgesia for mechanically ventilated critically ill patients: a prospective multicenter patient-based study. Anesthesiology. 2007; 106:687–95.
16. Wilhelm W, Kreuer S. The place for short-acting opioids: special emphasis on remifentanil. Crit Care. 2008; 12(Suppl 3):S5.

17. White PF, Kehlet H, Neal JM, Schricker T, Carr DB, Carli F, et al. The role of the anesthesiologist in fast-track surgery: from multimodal analgesia to perioperative medical care. Anesth Analg. 2007; 104:1380–96.

18. Cattabriga I, Pacini D, Lamazza G, Talarico F, Di Bartolomeo R, Grillone G, et al. Intravenous paracetamol as adjunctive treatment for postoperative pain after cardiac surgery: a double blind randomized controlled trial. Eur J Cardiothorac Surg. 2007; 32:527–31.

19. Beloeil H, Delage N, Nègre I, Mazoit JX, Benhamou D. The median effective dose of nefopam and morphine administered intravenously for postoperative pain after minor surgery: a prospective randomized double-blinded isobolographic study of their analgesic action. Anesth Analg. 2004; 98:395–400.

20. Pandey CK, Raza M, Tripathi M, Navkar DV, Kumar A, Singh UK. The comparative evaluation of gabapentin and carbamazepine for pain management in Guillain-Barré syndrome patients in the intensive care unit. Anesth Analg. 2005; 101:220–5.

21. Dasta JF, Fuhrman TM, McCandles C. Patterns of prescribing and administering drugs for agitation and pain in patients in a surgical intensive care unit. Crit Care Med. 1994; 22:974–80.

22. Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000; 342:1471–7.

23. Boldt J, Thaler E, Lehmann A, Papsdorf M, Isgro F. Pain management in cardiac surgery patients: comparison between standard therapy and patient-controlled analgesia regimen. J Cardiothorac Vasc Anesth. 1998; 12:654–8.

24. Blasco TA, Lee D, Amalric M, Swerdlow NR, Smith NT, Koob GF. The role of the nucleus raphe pontis and the caudate nucleus in alfentanil rigidity in the rat. Brain Res. 1986; 386:280–6.

25. Pokela ML, Ryhänen PT, Koivisto ME, Olkkola KT, Saukkonen AL. Alfentanil-induced rigidity in newborn infants. Anesth Analg. 1992; 75:252–7.

26. Bailey PL, Wilbrink J, Zwanikken P, Pace NL, Stanley TH. Anesthetic induction with fentanyl. Anesth Analg. 1985; 64:48–53.

27. Cantais A, Schnell D, Vincent F, Hammouda Z, Perinel S, Balichard S, et al. Acetaminophen-induced changes in systemic blood pressure in critically ill patients: results of a multicenter cohort study. Crit Care Med. 2016; 44:2192–8.
28. Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013; 41:263–306.

29. Kollef MH, Levy NT, Ahrens TS, Schaiff R, Prentice D, Sherman G. The use of continuous i.v. sedation is associated with prolongation of mechanical ventilation. Chest. 1998; 114:541–8.

30. Brattebø G, Hofoss D, Flaatten H, Muri AK, Gjerde S, Plsek PE. Effect of a scoring system and protocol for sedation on duration of patients’ need for ventilator support in a surgical intensive care unit. BMJ. 2002; 324:1386–9.
31. Shehabi Y, Bellomo R, Reade MC, Bailey M, Bass F, Howe B, et al. Early goal-directed sedation versus standard sedation in mechanically ventilated critically ill patients: a pilot study. Crit Care Med. 2013; 41:1983–91.
32. Bugedo G, Tobar E, Aguirre M, Gonzalez H, Godoy J, Lira MT, et al. The implementation of an analgesia-based sedation protocol reduced deep sedation and proved to be safe and feasible in patients on mechanical ventilation. Rev Bras Ter Intensiva. 2013; 25:188–96.

33. Carson SS, Kress JP, Rodgers JE, Vinayak A, Campbell-Bright S, Levitt J, et al. A randomized trial of intermittent lorazepam versus propofol with daily interruption in mechanically ventilated patients. Crit Care Med. 2006; 34:1326–32.

34. Mehta S, Burry L, Cook D, Fergusson D, Steinberg M, Granton J, et al. Daily sedation interruption in mechanically ventilated critically ill patients cared for with a sedation protocol: a randomized controlled trial. JAMA. 2012; 308:1985–92.

35. Riker RR, Fraser GL. Monitoring sedation, agitation, analgesia, neuromuscular blockade, and delirium in adult ICU patients. Semin Respir Crit Care Med. 2001; 22:189–98.

36. MacIntyre N. Discontinuing mechanical ventilatory support. Chest. 2007; 132:1049–56.

37. Vinik HR, Kissin I. Rapid development of tolerance to analgesia during remifentanil infusion in humans. Anesth Analg. 1998; 86:1307–11.

38. Boles JM, Bion J, Connors A, Herridge M, Marsh B, Melot C, et al. Weaning from mechanical ventilation. Eur Respir J. 2007; 29:1033–56.

39. Olson DM, Thoyre SM, Peterson ED, Graffagnino C. A randomized evaluation of bispectral index-augmented sedation assessment in neurological patients. Neurocrit Care. 2009; 11:20–7.

40. Yang KS, Habib AS, Lu M, Branch MS, Muir H, Manberg P, et al. A prospective evaluation of the incidence of adverse events in nurse-administered moderate sedation guided by sedation scores or Bispectral Index. Anesth Analg. 2014; 119:43–8.

41. Matić I, Majerić-Kogler V. Comparison of pressure support and T-tube weaning from mechanical ventilation: randomized prospective study. Croat Med J. 2004; 45:162–6.
42. Farias JA, Retta A, Alía I, Olazarri F, Esteban A, Golubicki A, et al. A comparison of two methods to perform a breathing trial before extubation in pediatric intensive care patients. Intensive Care Med. 2001; 27:1649–54.

43. Jones DP, Byrne P, Morgan C, Fraser I, Hyland R. Positive end-expiratory pressure vs T-piece: extubation after mechanical ventilation. Chest. 1991; 100:1655–9.
44. Haberthür C, Mols G, Elsasser S, Bingisser R, Stocker R, Guttmann J. Extubation after breathing trials with automatic tube compensation, T-tube, or pressure support ventilation. Acta Anaesthesiol Scand. 2002; 46:973–9.

45. Esteban A, Alía I, Tobin MJ, Gil A, Gordo F, Vallverdú I, et al. Effect of spontaneous breathing trial duration on outcome of attempts to discontinue mechanical ventilation. Spanish Lung Failure Collaborative Group. Am J Respir Crit Care Med. 1999; 159:512–8.

46. Perren A, Domenighetti G, Mauri S, Genini F, Vizzardi N. Protocol-directed weaning from mechanical ventilation: clinical outcome in patients randomized for a 30-min or 120-min trial with pressure support ventilation. Intensive Care Med. 2002; 28:1058–63.

47. Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE Jr, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004; 291:1753–62.

48. Vallverdú I, Calaf N, Subirana M, Net A, Benito S, Mancebo J. Clinical characteristics, respiratory functional parameters, and outcome of a two-hour T-piece trial in patients weaning from mechanical ventilation. Am J Respir Crit Care Med. 1998; 158:1855–62.

49. Brochard L, Rauss A, Benito S, Conti G, Mancebo J, Rekik N, et al. Comparison of three methods of gradual withdrawal from ventilatory support during weaning from mechanical ventilation. Am J Respir Crit Care Med. 1994; 150:896–903.

50. Koh WY, Lew TW, Chin NM, Wong MF. Tracheostomy in a neuro-intensive care setting: indications and timing. Anaesth Intensive Care. 1997; 25:365–8.

51. Snellen F, Lauwers P, Demeyere R, Byttebier G, Van Aken H. The use of midazolam versus propofol for short-term sedation following coronary artery bypass grafting. Intensive Care Med. 1990; 16:312–6.

52. Roekaerts PM, Huygen FJ, de Lange S. Infusion of propofol versus midazolam for sedation in the intensive care unit following coronary artery surgery. J Cardiothorac Vasc Anesth. 1993; 7:142–7.

53. Huey-Ling L, Chun-Che S, Jen-Jen T, Shau-Ting L, Hsing-I C. Comparison of the effect of protocol-directed sedation with propofol vs. midazolam by nurses in intensive care: efficacy, haemodynamic stability and patient satisfaction. J Clin Nurs. 2008; 17:1510–7.

54. Zhou Y, Jin X, Kang Y, Liang G, Liu T, Deng N. Midazolam and propofol used alone or sequentially for long-term sedation in critically ill, mechanically ventilated patients: a prospective, randomized study. Crit Care. 2014; 18:R122.

55. Sandiumenge Camps A, Sanchez-Izquierdo Riera JA, Toral Vazquez D, Sa Borges M, Peinado Rodriguez J, Alted Lopez E. Midazolam and 2% propofol in long-term sedation of traumatized critically ill patients: efficacy and safety comparison. Crit Care Med. 2000; 28:3612–9.

56. Mesnil M, Capdevila X, Bringuier S, Trine PO, Falquet Y, Charbit J, et al. Long-term sedation in intensive care unit: a randomized comparison between inhaled sevoflurane and intravenous propofol or midazolam. Intensive Care Med. 2011; 37:933–41.

57. Riker RR, Shehabi Y, Bokesch PM, Ceraso D, Wisemandle W, Koura F, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009; 301:489–99.

58. Jakob SM, Ruokonen E, Grounds RM, Sarapohja T, Garratt C, Pocock SJ, et al. Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: two randomized controlled trials. JAMA. 2012; 307:1151–60.

59. Sachdeva A, Choudhary M, Chandra M. Alcohol withdrawal syndrome: benzodiazepines and beyond. J Clin Diagn Res. 2015; 9:VE01-7.

60. Nutman J, Brooks LJ, Deakins KM, Baldesare KK, Witte MK, Reed MD. Racemic versus l-epinephrine aerosol in the treatment of postextubation laryngeal edema: results from a prospective, randomized, double-blind study. Crit Care Med. 1994; 22:1591–4.

61. Vitacca M, Vianello A, Colombo D, Clini E, Porta R, Bianchi L, et al. Comparison of two methods for weaning patients with chronic obstructive pulmonary disease requiring mechanical ventilation for more than 15 days. Am J Respir Crit Care Med. 2001; 164:225–30.

62. Benbenbishty J, Adam S, Endacott R. Physical restraint use in intensive care units across Europe: the PRICE study. Intensive Crit Care Nurs. 2010; 26:241–5.

63. Kandeel NA, Attia AK. Physical restraints practice in adult intensive care units in Egypt. Nurs Health Sci. 2013; 15:79–85.

64. Rose L, Burry L, Mallick R, Luk E, Cook D, Fergusson D, et al. Prevalence, risk factors, and outcomes associated with physical restraint use in mechanically ventilated adults. J Crit Care. 2016; 31:31–5.

65. Burry LD, Williamson DR, Perreault MM, Rose L, Cook DJ, Ferguson ND, et al. Analgesic, sedative, antipsychotic, and neuromuscular blocker use in Canadian intensive care units: a prospective, multicentre, observational study. Can J Anaesth. 2014; 61:619–30.

66. Dubois MJ, Bergeron N, Dumont M, Dial S, Skrobik Y. Delirium in an intensive care unit: a study of risk factors. Intensive Care Med. 2001; 27:1297–304.

67. Salam A, Tilluckdharry L, Amoateng-Adjepong Y, Manthous CA. Neurologic status, cough, secretions and extubation outcomes. Intensive Care Med. 2004; 30:1334–9.

68. Inouye SK. Delirium in older persons. N Engl J Med. 2006; 354:1157–65.

69. van den Boogaard M, Pickkers P, Slooter AJ, Kuiper MA, Spronk PE, van der Voort PH, et al. Development and validation of PRE-DELIRIC (PREdiction of DELIRium in ICu patients) delirium prediction model for intensive care patients: observational multicentre study. BMJ. 2012; 344:e420.

70. van den Boogaard M, Schoonhoven L, Maseda E, Plowright C, Jones C, Luetz A, et al. Recalibration of the delirium prediction model for ICU patients (PRE-DELIRIC): a multinational observational study. Intensive Care Med. 2014; 40:361–9.

71. Wassenaar A, van den Boogaard M, van Achterberg T, Slooter AJ, Kuiper MA, Hoogendoorn ME, et al. Multinational development and validation of an early prediction model for delirium in ICU patients. Intensive Care Med. 2015; 41:1048–56.

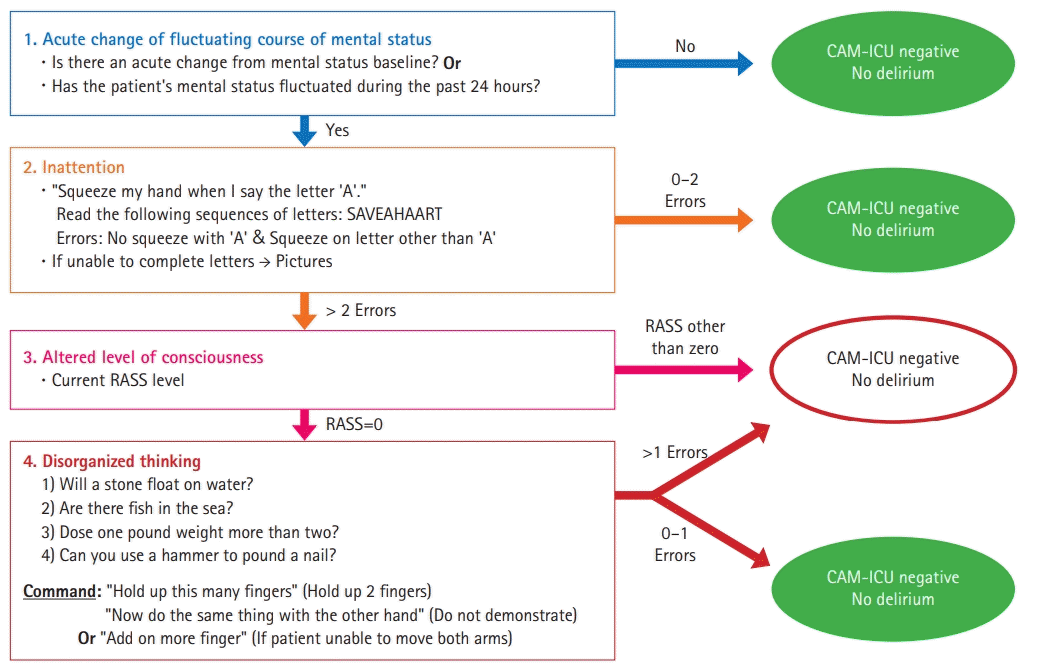
72. Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001; 286:2703–10.
73. Andrews L, Silva SG, Kaplan S, Zimbro K. Delirium monitoring and patient outcomes in a general intensive care unit. Am J Crit Care. 2015; 24:48–56.

74. Bigatello LM, Amirfarzan H, Haghighi AK, Newhouse B, Del Rio JM, Allen K, et al. Effects of routine monitoring of delirium in a surgical/trauma intensive care unit. J Trauma Acute Care Surg. 2013; 74:876–83.

75. Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013; 369:1306–16.

76. Girard TD, Pandharipande PP, Carson SS, Schmidt GA, Wright PE, Canonico AE, et al. Feasibility, efficacy, and safety of antipsychotics for intensive care unit delirium: the MIND randomized, placebo-controlled trial. Crit Care Med. 2010; 38:428–37.

77. Page VJ, Ely EW, Gates S, Zhao XB, Alce T, Shintani A, et al. Effect of intravenous haloperidol on the duration of delirium and coma in critically ill patients (Hope-ICU): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2013; 1:515–23.

78. Devlin JW, Roberts RJ, Fong JJ, Skrobik Y, Riker RR, Hill NS, et al. Efficacy and safety of quetiapine in critically ill patients with delirium: a prospective, multicenter, randomized, double-blind, placebo-controlled pilot study. Crit Care Med. 2010; 38:419–27.

79. Skrobik YK, Bergeron N, Dumont M, Gottfried SB. Olanzapine vs haloperidol: treating delirium in a critical care setting. Intensive Care Med. 2004; 30:444–9.

80. Needham DM, Colantuoni E, Dinglas VD, Hough CL, Wozniak AW, Jackson JC, et al. Rosuvastatin versus placebo for delirium in intensive care and subsequent cognitive impairment in patients with sepsis-associated acute respiratory distress syndrome: an ancillary study to a randomised controlled trial. Lancet Respir Med. 2016; 4:203–12.

81. Reade MC, Eastwood GM, Bellomo R, Bailey M, Bersten A, Cheung B, et al. Effect of dexmedetomidine added to standard care on ventilator-free time in patients with agitated delirium: a randomized clinical trial. JAMA. 2016; 315:1460–8.

82. Al-Qadheeb NS, Skrobik Y, Schumaker G, Pacheco MN, Roberts RJ, Ruthazer RR, et al. Preventing ICU subsyndromal delirium conversion to delirium with low-dose IV haloperidol: a double-blind, placebo-controlled pilot study. Crit Care Med. 2016; 44:583–91.
83. van den Boogaard M, Slooter AJ, Brüggemann RJ, Schoonhoven L, Beishuizen A, Vermeijden JW, et al. Effect of haloperidol on survival among critically ill adults with a high risk of delirium: the REDUCE Randomized Clinical Trial. JAMA. 2018; 319:680–90.

84. Skrobik Y, Duprey MS, Hill NS, Devlin JW. Low-dose nocturnal dexmedetomidine prevents ICU delirium: a randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2018; 197:1147–56.

85. Foster J, Kelly M. A pilot study to test the feasibility of a nonpharmacologic intervention for the prevention of delirium in the medical intensive care unit. Clin Nurse Spec. 2013; 27:231–8.

86. Moon KJ, Lee SM. The effects of a tailored intensive care unit delirium prevention protocol: a randomized controlled trial. Int J Nurs Stud. 2015; 52:1423–32.

87. Colombo R, Corona A, Praga F, Minari C, Giannotti C, Castelli A, et al. A reorientation strategy for reducing delirium in the critically ill: results of an interventional study. Minerva Anestesiol. 2012; 78:1026–33.
88. Hanison J, Conway D. A multifaceted approach to prevention of delirium on intensive care. BMJ Qual Improv Rep. 2015; 4:u209656.w4000.

89. Lee HJ, Bae E, Lee HY, Lee SM, Lee J. Association of natural light exposure and delirium according to the presence or absence of windows in the intensive care unit. Acute Crit Care. 2021; 36:332–41.

90. Barnes-Daly MA, Phillips G, Ely EW. Improving hospital survival and reducing brain dysfunction at seven California community hospitals: implementing PAD guidelines via the ABCDEF bundle in 6,064 patients. Crit Care Med. 2017; 45:171–8.
91. Denehy L, Lanphere J, Needham DM. Ten reasons why ICU patients should be mobilized early. Intensive Care Med. 2017; 43:86–90.

92. Fan E, Dowdy DW, Colantuoni E, Mendez-Tellez PA, Sevransky JE, Shanholtz C, et al. Physical complications in acute lung injury survivors: a two-year longitudinal prospective study. Crit Care Med. 2014; 42:849–59.
93. Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009; 373:1874–82.

94. Devlin JW, Skrobik Y, Gélinas C, Needham DM, Slooter AJ, Pandharipande PP, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018; 46:e825–73.
95. Dong ZH, Yu BX, Sun YB, Fang W, Li L. Effects of early rehabilitation therapy on patients with mechanical ventilation. World J Emerg Med. 2014; 5:48–52.

96. Burtin C, Clerckx B, Robbeets C, Ferdinande P, Langer D, Troosters T, et al. Early exercise in critically ill patients enhances short-term functional recovery. Crit Care Med. 2009; 37:2499–505.

97. Waldauf P, Jiroutková K, Krajčová A, Puthucheary Z, Duška F. Effects of rehabilitation interventions on clinical outcomes in critically ill patients: systematic review and meta-analysis of randomized controlled trials. Crit Care Med. 2020; 48:1055–65.
98. Chao PW, Shih CJ, Lee YJ, Tseng CM, Kuo SC, Shih YN, et al. Association of postdischarge rehabilitation with mortality in intensive care unit survivors of sepsis. Am J Respir Crit Care Med. 2014; 190:1003–11.

99. Abrams D, Javidfar J, Farrand E, Mongero LB, Agerstrand CL, Ryan P, et al. Early mobilization of patients receiving extracorporeal membrane oxygenation: a retrospective cohort study. Crit Care. 2014; 18:R38.

100. Mayer KP, Joseph-Isang E, Robinson LE, Parry SM, Morris PE, Neyra JA. Safety and feasibility of physical rehabilitation and active mobilization in patients requiring continuous renal replacement therapy: a systematic review. Crit Care Med. 2020; 48:e1112–20.

101. Sricharoenchai T, Parker AM, Zanni JM, Nelliot A, Dinglas VD, Needham DM. Safety of physical therapy interventions in critically ill patients: a single-center prospective evaluation of 1110 intensive care unit admissions. J Crit Care. 2014; 29:395–400.

102. Nydahl P, Sricharoenchai T, Chandra S, Kundt FS, Huang M, Fischill M, et al. Safety of patient mobilization and rehabilitation in the intensive care unit: systematic review with meta-analysis. Ann Am Thorac Soc. 2017; 14:766–77.

103. Kim W. Rehabilitation in intensive care unit. J Acute Care Surg. 2018; 8:2–6.

104. Cooper AB, Thornley KS, Young GB, Slutsky AS, Stewart TE, Hanly PJ. Sleep in critically ill patients requiring mechanical ventilation. Chest. 2000; 117:809–18.

105. Mundigler G, Delle-Karth G, Koreny M, Zehetgruber M, Steindl-Munda P, Marktl W, et al. Impaired circadian rhythm of melatonin secretion in sedated critically ill patients with severe sepsis. Crit Care Med. 2002; 30:536–40.

106. Cabello B, Thille AW, Drouot X, Galia F, Mancebo J, d’Ortho MP, et al. Sleep quality in mechanically ventilated patients: comparison of three ventilatory modes. Crit Care Med. 2008; 36:1749–55.

107. Van Rompaey B, Elseviers MM, Van Drom W, Fromont V, Jorens PG. The effect of earplugs during the night on the onset of delirium and sleep perception: a randomized controlled trial in intensive care patients. Crit Care. 2012; 16:R73.

108. Patel J, Baldwin J, Bunting P, Laha S. The effect of a multicomponent multidisciplinary bundle of interventions on sleep and delirium in medical and surgical intensive care patients. Anaesthesia. 2014; 69:540–9.

109. Alexopoulou C, Kondili E, Diamantaki E, Psarologakis C, Kokkini S, Bolaki M, et al. Effects of dexmedetomidine on sleep quality in critically ill patients: a pilot study. Anesthesiology. 2014; 121:801–7.
110. Oto J, Yamamoto K, Koike S, Onodera M, Imanaka H, Nishimura M. Sleep quality of mechanically ventilated patients sedated with dexmedetomidine. Intensive Care Med. 2012; 38:1982–9.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download