Abstract
Background
In septic shock patients with cirrhosis, impaired liver function might decrease lactate elimination and produce a higher lactate level. This study investigated differences in initial lactate, lactate clearance, and lactate utility between cirrhotic and non-cirrhotic septic shock patients.
Methods
This is a retrospective cohort study conducted at a referral, university-affiliated medical center. We enrolled adults admitted during 2012–2018 who satisfied the septic shock diagnostic criteria of the Surviving Sepsis Campaign: 2012. Patients previously diagnosed with cirrhosis by an imaging modality were classified into the cirrhosis group. The initial lactate levels and levels 6 hours after resuscitation were measured and used to calculate lactate clearance. We compared initial lactate, lactate at 6 hours, and lactate clearance between the cirrhosis and non-cirrhosis groups. The primary outcome was in-hospital mortality.
Results
Overall 777 patients were enrolled, of whom 91 had previously been diagnosed with cirrhosis. Initial lactate and lactate at 6 hours were both significantly higher in cirrhosis patients, but there was no difference between the groups in lactate clearance. A receiver operating characteristic curve analysis for predictors of in-hospital mortality revealed cut-off values for initial lactate, lactate at 6 hours, and lactate clearance of >4 mmol/L, >2 mmol/L, and <10%, respectively, among non-cirrhosis patients. Among patients with cirrhosis, the cut-off values predicting in-hospital mortality were >5 mmol/L, >5 mmol/L, and <20%, respectively. Neither lactate level nor lactate clearance was an independent risk factor for in-hospital mortality among cirrhotic and non-cirrhotic septic shock patients.
Septic shock is a life-threatening condition that is a major healthcare burden worldwide. The reported mortality from septic shock ranges from 42% to 53% [1-3]. To improve outcomes, early diagnosis, appropriate antibiotics, infectious source drainage, and rapid hemodynamic restoration should be provided. The serum lactate level has been added as a component parameter of the criteria used to diagnose septic shock, and it is also used to guide tissue perfusion restoration. Previously reported evidence suggested that the serum lactate level might be an independent factor associated with poor septic shock management outcomes.
Recently proposed diagnostic criteria (sepsis and septic shock [Sepsis-3]) recommend that septic shock be diagnosed if a patient has clinical features of sepsis with hypotension that requires vasopressors to maintain a mean arterial blood pressure (mABP) of 65 mm Hg or higher and has a serum lactate level of 2 mmol/L or more despite adequate volume resuscitation [4]. Using those criteria, in-hospital mortality is in excess of 40%. The decision to include lactate levels in the septic shock diagnostic criteria was based on a systematic review of 44 epidemiologic studies reporting septic shock outcomes. A previous study that influenced the decision to include serum lactate levels in the diagnostic criteria for septic shock reported an increase in the adjusted odds ratio [OR] for in-hospital mortality from 1.4 (95% confidence interval [CI], 1.35–1.45) to 3.03 (95% CI, 2.68–3.45) as the serum lactate level rose from 2 to 10 mmol/L [1]. Treatment involves initial fluid resuscitation and vasopressor administration to maintain adequate perfusion pressure, with additional treatment guided by frequent reassessments of hemodynamic status to achieve adequate tissue perfusion. Reassessments should include physical examinations, urine output, and serum lactate levels [5-7]. Previous studies reported an association between early lactate clearance and reduced in-hospital mortality among septic shock patients [8-10].
The serum lactate level, one of the most important markers in septic shock patients, depends on the lactate production and elimination rates. Increased serum lactate levels occur mainly in situations that cause increased lactate production such as shock, which causes anaerobic metabolism due to inadequate tissue perfusion (type A lactic acidosis). However, deterioration in lactate elimination can also cause increasing serum lactate (type B lactic acidosis), and that condition has been associated with malignancy and the use of specific medications [11-14]. The liver plays the major role in lactate elimination, so impaired liver function could decrease it [11]. Therefore, in septic shock patients with documented cirrhosis, impaired liver function might result in higher lactate levels, impaired lactate clearance, and delayed normalization of lactate during septic shock. There is currently a scarcity of information specific to differences in serum lactate levels and lactate clearance between cirrhotic and non-cirrhotic septic shock patients.
Therefore, the primary objective of this study was to identify differences in serum lactate levels and lactate clearance between cirrhotic and non-cirrhotic septic shock patients. The secondary objective was to evaluate the utility of lactate levels and lactate clearance in predicting in-hospital mortality among cirrhotic and non-cirrhotic septic shock patients.
This study protocol was approved by the Institutional Review Board of Siriraj Hospital (IRB No. 421/2018). The requirement to obtain informed consent was waived due to our study’s retrospective design.
This retrospective cohort study was conducted at the medical intensive care unit of a referral, university-affiliated medical center. The study included adult patients (at least 18 years old) who were admitted between January 2012 and June 2018 and met the criteria for a septic shock diagnosis according to the Surviving Sepsis Campaign: International Guidelines for Management of Severe Sepsis and Septic Shock: 2012 [15]. Septic shock was defined as hypotension with an mABP lower than 65 mm Hg or requiring a vasopressor to maintain mABP ≥65 mm Hg and the presence of a documented infection with evidence of systemic inflammation. Patients with prolonged shock (longer than 24 hours), do-not-resuscitate orders, pregnancy, or a lack of serum lactate level data at baseline or follow-up were excluded. Every septic shock patient was resuscitated according to a septic shock protocol that includes fluid resuscitation, vasopressor therapy, antimicrobial initiation within 1 hour, appropriate source control, and organ support.
We performed an electronic medical records review from which we collected and recorded patient baseline characteristics: age, sex, underlying conditions, severity score, and vital signs. The baseline serum lactate level was defined as the first serum lactate measurement from an arterial or venous blood sample [16] after the septic shock diagnosis. According to the septic shock diagnostic criteria used during the study period, serum lactate was measured within 3 hours after the septic shock diagnosis. To calculate serum lactate clearance, we used the following formula: [(initial serum lactate level–follow-up serum lactate)/initial serum lactate×100]. At our center, follow-up serum lactate measurements were performed after patients were resuscitated and achieved the macro-circulation goal of mABP ≥65 mm Hg, usually 6 hours after the initiation of resuscitation. Data specific to the amount of fluid resuscitation, vasopressor type and dosage, organ support requirements, and treatment outcomes were also recorded. Patients were classified into the cirrhosis septic shock group if they had been diagnosed with cirrhosis using evidence obtained from ultrasonography, computerized tomography, or magnetic resonance imaging before they developed septic shock. Patients without a previous diagnosis of cirrhosis were classified into the non-cirrhosis group. The primary outcome of this study was in-hospital mortality.
Continuous data are expressed as the mean±standard deviation, and categorical data are expressed as percentages. Independent t-testing was used to compare continuous variables with a normal distribution. For the comparison of non-normally distributed continuous variables, the Mann-Whitney U-test was used. The chi-square test or Fischer’s exact test was used to compare categorical variables.
To identify predictive factors associated with in-hospital mortality, patients were classified into non-cirrhosis and cirrhosis groups. A comparative analysis was then performed between the survivors and non-survivors in each group. A receiver operating characteristic (ROC) curve analysis was performed to identify cut-off values for the continuous variables that differed significantly between the survivors and non-survivors in each group. Each optimal cut-off value, along with its sensitivity and specificity, was determined using Youden’s index [17]. The largest area under the ROC curve (AUC) was used to identify which variable (initial lactate, lactate at 6 hours, or lactate clearance) best predicted in-hospital mortality. Variables were reclassified into two groups using the ROC curve–identified cut-off values, and then univariate analyses were performed. Risk is expressed as an unadjusted OR with the 95% CI. Predictive factors with a P-value equal to or less than 0.1 and other factors of interest were enrolled in binary logistic regression analyses. The results of the multivariate analysis are shown as adjusted OR with the 95% CI and P-value. Independent predictive factors associated with in-hospital mortality are those with a P-value equal to or less than 0.05. All statistical analyses were performed using SPSS ver. 18 (SPSS Inc., Chicago, IL, USA).
A total of 777 septic shock patients were enrolled in this study. Of them, 91 patients (11.7%) had been diagnosed with cirrhosis by radiological imaging before they developed septic shock. The demographic and clinical characteristics of the study participants are shown in Table 1. The mean age of the patients was 63.8±16.4 years, and 50.6% of them were male. There was no significant difference in the baseline APACHE II score or mABP between the cirrhosis and non-cirrhosis patients. However, the cirrhotic septic shock patients had significantly higher body mass index, lower temperature, and slower heart rate than the non-cirrhosis patients. The most common infection in the non-cirrhosis patients was pneumonia (34.7%). Intra-abdominal infections were the most common infection among cirrhosis patients (39.6%).
Cirrhosis patients received a larger volume of fluid resuscitation during days 2 and 3 after septic shock than the non-cirrhosis patients. The maximum vasopressor dose did not differ between the groups, but the cirrhosis patients required adrenaline and dopamine in higher proportions. Twenty-eight-day mortality was significantly higher among patients with both sepsis and cirrhosis than among patients without cirrhosis (34.1% vs. 24.1%; P=0.05); however, in-hospital mortality did not differ significantly between the groups (36.3% vs. 30.1%; P=0.23) (Table 1).
Regarding serum lactate levels (90% of cases reported venous lactate levels), the initial serum lactate level (7.5±6.1 vs. 4.3±3.7 mmol/L; P<0.001) and serum lactate at 6 hours (4.5±4.1 vs. 3.2±3.0 mmol/L; P=0.01) were both significantly higher among patients with both septic shock and cirrhosis than among patients without cirrhosis. Lactate clearance, however, did not differ significantly between the cirrhosis and non-cirrhosis groups (27.3%±24.7% vs. 23.5%±21.5%; P=0.35). In the non-cirrhotic septic shock patients, initial serum lactate (5.6±5.1 vs. 3.8±2.8 mmol/L; P<0.001) and lactate at 6 hours (4.1±4.3 vs. 2.8±2.0 mmol/L; P<0.001) were significantly higher, and lactate clearance was significantly lower (20.0%±20.4% vs. 25.1%±21.8%; P=0.02) among patients who died in the hospital than in those who survived. In the cirrhotic septic shock patients, only the initial serum lactate level was significantly higher among non-survivors than among survivors (9.6±5.8 vs. 6.9±5.9 mmol/L; P=0.01) (Table 2).
When we considered the influence of age and lactate levels in subgroup analyses of patients aged 18 to <60 years old, 60 to <80 years old, and ≥80 years old, we found no significant differences in the initial serum lactate level, serum lactate at 6 hours, or lactate clearance between the non-cirrhosis and cirrhosis groups in any age group (Table 3). Patients who required renal replacement therapy (RRT) had a significantly higher initial serum lactate level (7.4±6.2 vs. 4.2±3.5 mmol/L; P<0.001) and serum lactate at 6 hours (5.5±5.4 vs. 2.9±2.2 mmol/L; P<0.001) than patients who did not require RRT, and lactate clearance was significantly lower among patients who required RRT (18.5%±19.1% vs. 24.9%±22.1%; P=0.02) (Table 3).
To identify serum lactate and lactate clearance cut-off values that predict in-hospital survival, we used a ROC curve analysis. The AUC and optimal cut-off values (according to Youden’s index) for each lactate parameter are shown in Figure 1 and Supplementary Table 1. In non-cirrhotic septic shock patients, an initial serum lactate level of 4 mmol/L or more, a lactate level at 6 hours of 2 mmol/L or more, and lactate clearance of 10% or less predicted in-hospital mortality. In cirrhotic septic shock patients, an initial serum lactate level of 5 mmol/L or more, a lactate level at 6 hours of 5 mmol/L or more, and lactate clearance of 20% or less were identified as cut-off values, but they showed no association with in-hospital mortality.
Table 4 shows the independent predictive factors for in-hospital mortality among non-cirrhotic septic shock patients. Our multivariate analysis model included 17 mortality-associated parameters identified in the univariate analyses with a P-value <0.1. The multivariate analysis revealed that an APACHE II score ≥20, maximum vasopressor dose ≥0.2 µg/kg/min, pneumonia, bacteremia, requirement for RRT, and need for mechanical ventilator support were predictive factors associated with in-hospital mortality. That same analysis revealed that a body mass index ≥21 kg/m2 and urinary tract infection were protective factors against in-hospital mortality.
In cirrhotic septic shock patients, 13 parameters were found to be significantly associated with in-hospital mortality in the univariate analyses. The multivariate model identified a maximum vasopressor dose ≥0.2 µg/kg/min and the need for mechanical ventilator support as independent predictive factors associated with in-hospital mortality (Table 5).
Because the relationships among the initial serum lactate level, serum lactate at 6 hours, and lactate clearance could interfere with the result of a multivariate analysis, we analyzed the data by entering those three lactate parameters one-by-one. Even so, we did not identify any of them as an independent predictive factor associated with in-hospital mortality in either the non-cirrhosis or cirrhosis groups.
The results of this study show that the initial lactate levels and lactate at 6 hours were both significantly higher among cirrhotic than non-cirrhotic septic shock patients. However, lactate clearance did not differ significantly between the groups. Twenty-eight-day mortality was significantly higher among cirrhosis patients, but in-hospital mortality did not differ significantly between the groups. For septic shock prognostic determination, baseline serum lactate was significantly higher among patients who died in the hospital than among those who survived in both the cirrhosis and non-cirrhosis groups. Our multivariate analyses revealed no independent association between any of the three lactate parameters we investigated and in-hospital mortality in either the cirrhotic or non-cirrhotic septic shock groups.
The elevation of lactate level in septic shock patients results from both an overproduction of lactate and decreasing lactate elimination. During shock, tissue hypoxemia occurs due to inadequate blood flow. That condition inhibits aerobic metabolism via Kreb’s cycle, which results in the overproduction of lactate, the metabolic end product of anaerobic glycolysis. The liver is the major organ responsible for lactate elimination [11]. Impaired liver function through either chronic or acute processes could result in delayed lactate clearance. In septic shock, liver dysfunction has been reported as a complication associated with poor outcomes [4]. Therefore, the elevation of lactate from the combination of overproduction and under elimination might reflect the severity of septic shock. Among cirrhosis patients without sepsis, evidence of elevated lactate levels has been found in blood samples from both the hepatic vein and the femoral artery. Furthermore, the lactate level correlated directly with portal pressure and the severity of liver dysfunction [18]. In patients with both septic shock and cirrhosis, a previous study reported that lactate clearance was delayed compared with lactate clearance in non-cirrhosis patients [19,20]. However, those studies did not report the baseline serum lactate levels or the lactate levels 6 hours after resuscitation. Our study demonstrated that septic shock patients with underlying cirrhosis had significantly higher baseline serum lactate levels and significantly higher lactate 6 hours after septic shock resuscitation than non-cirrhotic septic shock patients. Given that the initial mABP did not differ significantly between the groups, the observed higher lactate levels in the cirrhotic septic shock patients could be associated with impaired liver clearance rather than lactate overproduction from tissue hypoxemia caused by shock. Given the higher adrenaline requirement we found among cirrhosis patients, another explanation for the higher serum lactate levels among cirrhosis patients could be overt aerobic glycolysis secondary to β-2 adrenergic receptor stimulation during adrenaline infusion [11]. However, that might not be the major cause of the lactate difference because only 24.2% of the cirrhosis patients received adrenaline during septic shock resuscitation.
Information from the Surviving Sepsis Campaign database indicates that a lactate level greater than 4 mmol/L is an independent predictor of septic shock mortality [21]. Other studies found that higher lactate levels were associated with higher septic shock mortality. The recently proposed Sepsis-3 definition, “lactate level greater than 2 mmol/L together with a requirement for vasopressor to maintain mABP in the absence of volume depletion,” was used in our study as the clinical criteria for diagnosing septic shock [4]. Using that lower lactate level cut-off value improves the sensitivity for diagnosis; however, it might also limit the specificity in predicting a poor prognosis. Because it had the largest AUC, the initial serum lactate might be a better indicator than the lactate level at 6 hours or lactate clearance for predicting in-hospital mortality in both the cirrhosis and non-cirrhosis groups (Table 3). Using the highest Youden’s index value, an initial serum lactate level of greater than 4 mmol/L for non-cirrhosis patients and greater than 5 mmol/L for cirrhosis patients could be used as cut-off values for predicting in-hospital mortality among septic shock patients. Our multivariate analysis did not identify initial lactate, lactate at 6 hours, or lactate clearance as independent predictors of in-hospital mortality in either the cirrhotic or non-cirrhotic septic shock group. That might reflect the fact that we included multiple parameters that represent patient hemodynamic status and the severity of septic shock—baseline blood pressure, APACHE II score, day 1 fluid requirement, and maximum vasopressor dose—in our multivariate analysis model.
In contrast to previous studies that reported that lactate clearance was delayed among patients with both cirrhosis and septic shock compared with non-cirrhosis patients, the results of our study do not support that finding. Lactate clearance was actually higher in our cirrhotic septic shock patients than in our non-cirrhotic septic shock patients, but the difference between groups was not statistically significant. Furthermore, lactate clearance was not found to be a significant predictor of septic shock outcomes among cirrhosis and non-cirrhosis patients (Tables 4 and 5). The results of our subgroup analyses showed that both cirrhosis and non-cirrhosis patients who required RRT had significantly higher initial serum lactate levels and serum lactate at 6 hours than those who did not require RRT, and lactate clearance was significantly lower among patients who required RRT. Notably, cirrhosis patients who required RRT did not have significantly lower lactate clearance than non-cirrhosis patients who required RRT, and cirrhosis patients with no RRT did not have significantly higher lactate clearance than non-cirrhosis patients with no RRT. These findings suggest that preserved renal function plays an important role in lactate clearance among resuscitated septic shock patients, especially those with cirrhosis. Another possible explanation for the high lactate clearance observed among our cirrhosis patients was the higher fluid volume that they received during resuscitation because cirrhosis patients received slightly more fluid than non-cirrhosis patients. However, the difference in fluid resuscitation volume between groups on the 1st day of treatment was not significant.
Given the lack of association between liver function testing, including alanine transaminase, aspartate transaminase, alkaline phosphatase, and bilirubin levels, and the severity of liver disease, we decided to use a radiologically confirmed diagnosis of cirrhosis in this study. However, some asymptomatic cirrhosis patients who had no documented radiological imaging might have been allocated to the non-cirrhosis group. We did not use abnormal liver function tests to classify patients into the liver disease group because a certain proportion of non-cirrhosis sepsis patients can also have abnormal liver function tests [4].
We also observed that cirrhosis patients had a lower proportion of underlying hypertension, coronary artery disease, and stroke than the non-cirrhosis patients. The heart rates of cirrhosis patients were also significantly lower than in the non-cirrhosis group. Those findings could be explained by the hyperdynamic circulation associated with arterial vasodilatation, cirrhosis cardiomyopathy, and autonomic dysfunction. Arterial vasodilation is believed to be due to portosystemic shunting and bacterial translocation that produce redistribution of the blood volume, increased splanchnic blood flow, and decreased systemic vascular resistance [22]. The leading source of infection among cirrhosis patients was intra-abdominal infection, whereas it was pneumonia in non-cirrhosis patients. The higher proportion of intra-abdominal infection among cirrhosis patients has been reported by several studies [23,24]. Immune dysfunction combined with portosystemic shunting prevents gut-derived bacteria and their toxins from being cleared from portal circulation by the liver, which could be the main reason for an increased risk of infection among cirrhosis patients [25].
The limitations of this study should be mentioned. First, the diagnosis of cirrhosis using prior radiological imaging might have excluded patients with early cirrhosis, which could produce contamination bias. Considering that cirrhosis patients experience a range of severity from mild to severe dysfunction, the unavailability of data about baseline liver function before patients developed septic shock could be a related limitation of this study. Second, recent updates in the definition of septic shock might have produced discrepancies between the results of pre-definition-update study populations and current septic shock patients. Third, a certain proportion of patients could not achieve an mABP of 65 mm Hg or more 6 hours after the initiation of resuscitation. Thus, the lactate level reported at 6 hours that we used in our analyses might not reflect the restoration of microcirculation among those patients (and might explain our finding of no association between lactate clearance and septic shock outcomes in our study population). Fourth, the data from this study came from a single center that is also a national tertiary referral center that often receives complex cases that cannot be managed in a less sophisticated healthcare setting. As a result, the findings of this study might not be generalizable to other healthcare settings.
▪ The initial serum lactate level and serum lactate at 6 hours were significantly higher in cirrhotic septic shock patients than in non-cirrhotic septic shock patients. However, lactate clearance did not differ significantly between the groups.
▪ Cut-off values for initial lactate, lactate at 6 hours, and lactate clearance of >4 mmol/L, >2 mmol/L, and <10%, respectively, were identified as predictors of in-hospital mortality among non-cirrhosis patients.
▪ Among those with cirrhosis, the cut-off values predicting in-hospital mortality were >5 mmol/L, >5 mmol/L, and <20%, respectively.
▪ None of the three evaluated serum lactate parameters was found to be an independent predictor of in-hospital mortality in either study group.
ACKNOWLEDGMENTS
This study was supported by Siriraj Critical Care Research Funding.
The authors gratefully acknowledge Mr. Kevin P. Jones for editing this manuscript.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.4266/acc.2021.00332.
Supplemental Table 1.
ROC curve analysis to identify the serum lactate level cut-off value for predicting hospital mortality
REFERENCES
1. Kethireddy S, Bilgili B, Sees A, Kirchner HL, Ofoma UR, Light RB, et al. Culture-negative septic shock compared with culture-positive septic shock: a retrospective cohort study. Crit Care Med. 2018; 46:506–12.
2. Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS, et al. Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016; 315:775–87.

3. Permpikul C, Tongyoo S, Ratanarat R, Wilachone W, Poompichet A. Impact of septic shock hemodynamic resuscitation guidelines on rapid early volume replacement and reduced mortality. J Med Assoc Thai. 2010; 93 Suppl 1:S102–9.
4. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016; 315:801–10.

5. Jones AE, Shapiro NI, Trzeciak S, Arnold RC, Claremont HA, Kline JA, et al. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA. 2010; 303:739–46.

6. Permpikul C, Sringam P, Tongyoo S. Therapeutic goal achievements during severe sepsis and septic shock resuscitation and their association with patients' outcomes. J Med Assoc Thai. 2014; 97 Suppl 3:S176–83.
7. Permpikul C, Tongyoo S, Viarasilpa T, Trainarongsakul T, Chakorn T, Udompanturak S. Early use of norepinephrine in septic shock resuscitation (CENSER): a randomized trial. Am J Respir Crit Care Med. 2019; 199:1097–105.

8. Jansen TC, van Bommel J, Schoonderbeek FJ, Sleeswijk Visser SJ, van der Klooster JM, Lima AP, et al. Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med. 2010; 182:752–61.

9. Ryoo SM, Lee J, Lee YS, Lee JH, Lim KS, Huh JW, et al. Lactate level versus lactate clearance for predicting mortality in patients with septic shock defined by sepsis-3. Crit Care Med. 2018; 46:e489–95.

10. Promsin P, Grip J, Norberg Å, Wernerman J, Rooyackers O. Optimal cut-off for hourly lactate reduction in ICU-treated patients with septic shock. Acta Anaesthesiol Scand. 2019; 63:885–94.

12. Fall PJ, Szerlip HM. Lactic acidosis: from sour milk to septic shock. J Intensive Care Med. 2005; 20:255–71.

13. Fuller BM, Dellinger RP. Lactate as a hemodynamic marker in the critically ill. Curr Opin Crit Care. 2012; 18:267–72.

14. Garcia-Alvarez M, Marik P, Bellomo R. Sepsis-associated hyperlactatemia. Crit Care. 2014; 18:503.

15. Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013; 41:580–637.

16. Pattharanitima P, Tongyoo S, Ratanarat R, Wilachone W, Poompichet A, Permpikul C. Correlation of arterial, central venous and capillary lactate levels in septic shock patients. J Med Assoc Thai. 2011; 94 Suppl 1:S175–80.
18. Jeppesen JB, Mortensen C, Bendtsen F, Møller S. Lactate metabolism in chronic liver disease. Scand J Clin Lab Invest. 2013; 73:293–9.

19. Sterling SA, Puskarich MA, Jones AE. The effect of liver disease on lactate normalization in severe sepsis and septic shock: a cohort study. Clin Exp Emerg Med. 2015; 2:197–202.

20. Ha TS, Shin TG, Jo IJ, Hwang SY, Chung CR, Suh GY, et al. Lactate clearance and mortality in septic patients with hepatic dysfunction. Am J Emerg Med. 2016; 34:1011–5.

21. Casserly B, Phillips GS, Schorr C, Dellinger RP, Townsend SR, Osborn TM, et al. Lactate measurements in sepsis-induced tissue hypoperfusion: results from the Surviving Sepsis Campaign database. Crit Care Med. 2015; 43:567–73.
22. Møller S, Bendtsen F. The pathophysiology of arterial vasodilatation and hyperdynamic circulation in cirrhosis. Liver Int. 2018; 38:570–80.

23. Fernández J, Navasa M, Gómez J, Colmenero J, Vila J, Arroyo V, et al. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology. 2002; 35:140–8.

Figure 1.
Receiver operative characteristic (ROC) curve analysis to identify the serum lactate level cut-off value predicting in-hospital mortality. (A) ROC curve for non-cirrhosis patients. (B) ROC curve for cirrhosis patients. AUC: area under the ROC curve; CI: confidence interval.
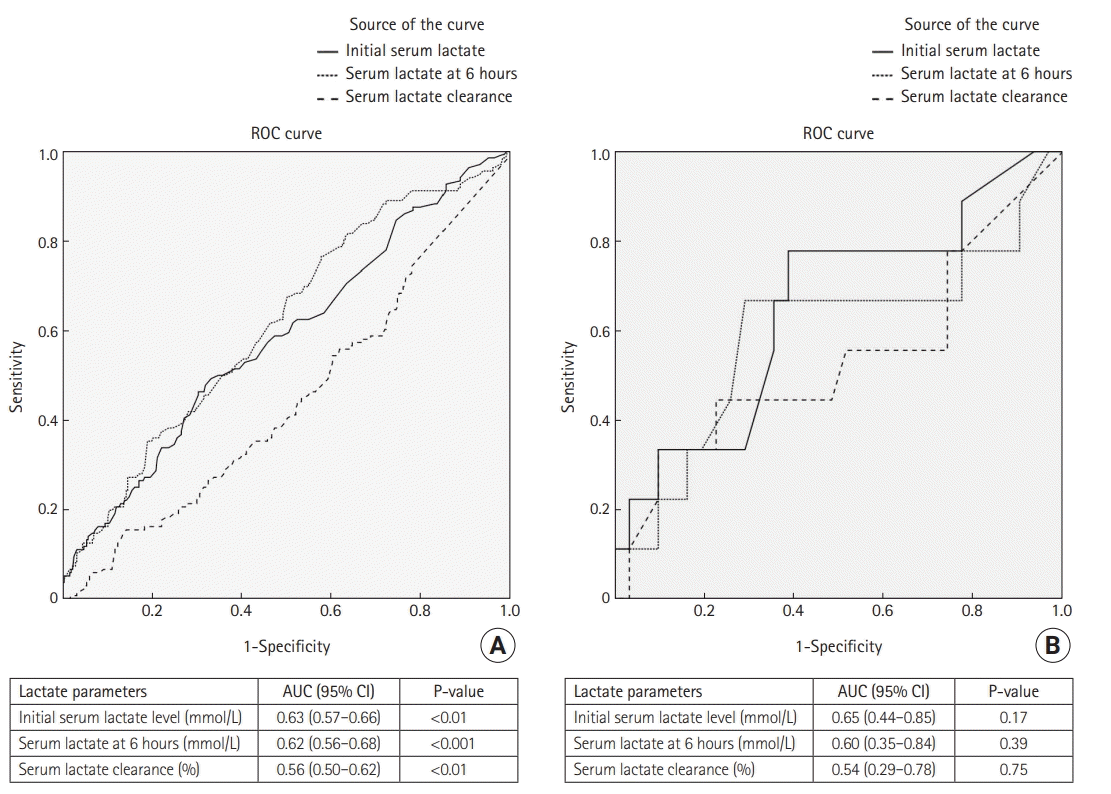
Table 1.
Demographic and baseline characteristics of study participants
| Characteristics | All (n=777) | Non-cirrhosis (n=686) | Cirrhosis (n=91) | P-value |
|---|---|---|---|---|
| Age (yr) | 63.8±16.4 | 64.0±16.0 | 62.8±11.9 | 0.51 |
| Body mass index (kg/m2) | 22.7±5.0 | 22.4±4.8 | 25.0±6.4 | <0.01 |
| Male (%) | 50.6 | 49.7 | 57.1 | 0.22 |
| APACHE II scorea | 21.6±7.3 | 21.5±7.2 | 22.3±7.7 | 0.34 |
| Temperature (ºC) | 37.8±1.4 | 37.8±1.4 | 37.4±1.3 | 0.02 |
| Heart rate (beats/min) | 108.4±25.0 | 109.7±24.7 | 98.7±24.8 | <0.01 |
| Respiratory rate (/min) | 26.6±6.6 | 26.8±6.5 | 25.6±6.9 | 0.12 |
| Mean arterial blood pressure (mm Hg) | 59.0±11.8 | 58.9±11.6 | 59.9±13.6 | 0.46 |
| Underlying disease (%) | ||||
| Hypertension | 48.6 | 50.4 | 35.2 | <0.01 |
| Diabetes mellitus | 36.0 | 36.3 | 34.1 | 0.73 |
| Coronary artery disease | 14.9 | 15.9 | 7.7 | 0.04 |
| Stroke | 11.1 | 12.2 | 2.2 | <0.01 |
| Chronic kidney disease | 17.0 | 16.9 | 17.6 | 0.88 |
| Malignancy | 22.7 | 22.7 | 22.0 | 1.00 |
| Source of infection (%) | ||||
| Pneumonia | 32.7 | 34.7 | 17.6 | <0.01 |
| Urinary tract infection | 24.6 | 25.8 | 15.4 | 0.04 |
| Intra-abdominal infection | 18.3 | 15.5 | 39.6 | <0.001 |
| Skin/soft tissue infection | 8.6 | 8.7 | 7.7 | 0.85 |
| Bacteremia | 19.4 | 18.2 | 28.6 | 0.02 |
| Treatment received | ||||
| Fluid day 1 (ml) | 5,401.2±2,036.5 | 5,375.1±2,042.5 | 5,597.4±1,991.3 | 0.33 |
| Fluid day 2 (ml) | 2,045.6±1,588.7 | 1,998.1±1,549.4 | 2,422.3±1,820.8 | 0.04 |
| Fluid day 3 (ml) | 1,548.5±1,527.7 | 1,504.5±1,501.2 | 1,877.4±1,685.5 | 0.05 |
| Norepinephrine (%) | 81.3 | 80.5 | 87.9 | 0.11 |
| Adrenaline (%) | 16.6 | 15.6 | 24.2 | 0.05 |
| Dopamine (%) | 28.3 | 26.2 | 44.0 | <0.01 |
| Dobutamine (%) | 4.1 | 4.3 | 1.7 | 0.50 |
| Maximum dose of vasopressorb (µg/kg/min) | 0.40±0.62 | 0.41±0.63 | 0.33±0.47 | 0.23 |
| Renal replacement therapy (%) | 14.9 | 14.3 | 19.8 | 0.16 |
| Ventilatory support (%) | 61.6 | 62.2 | 57.1 | 0.36 |
| Initial serum lactate level (mmol/L) | 4.7±4.2 | 4.3±3.7 | 7.5±6.1 | <0.001 |
| Serum lactate at 6 hours (mmol/L) | 3.3±3.1 | 3.2±3.0 | 4.5±4.1 | 0.01 |
| Serum lactate clearance (%) | 23.9±21.8 | 23.5±21.5 | 27.3±24.7 | 0.35 |
| In-hospital mortality (%) | 30.8 | 30.1 | 36.3 | 0.23 |
| 28-Day mortality (%) | 25.3 | 24.1 | 34.1 | 0.05 |
Table 2.
Serum lactate parameters of the non-cirrhosis and cirrhosis groups
Table 3.
Lactate parameters according to specific subgroups
| Characteristics | All (n=777) | Non-cirrhosis (n=686) | Cirrhosis (n=91) | P-value |
|---|---|---|---|---|
| Age groupa | ||||
| ≥80 yr | (n=133) | (n=127) | (n=6) | |
| Initial serum lactate level (mmol/L) | 4.4±3.4 | 4.3±3.4 | 5.6±3.6 | 0.44 |
| Serum lactate at 6 hours (mmol/L) | 2.9±2.1 | 3.0±2.2 | 2.3±0.5 | 0.54 |
| Serum lactate clearance (%) | 24.8±22.4 | 24.3±22.0 | 34.1±32.1 | 0.40 |
| 60–79 yr | (n=374) | (n=323) | (n=51) | |
| Initial serum lactate level (mmol/L) | 4.6±3.6 | 4.3±3.3 | 6.4±4.5 | <0.001 |
| Serum lactate at 6 hours (mmol/L) | 3.3±2.4 | 3.2±2.3 | 4.6±3.4 | <0.01 |
| Serum lactate clearance (%) | 24.0±21.9 | 23.8±21.5 | 26.0±26.2 | 0.67 |
| 18–59 yr | (n=270) | (n=236) | (n=34) | |
| Initial serum lactate level (mmol/L) | 5.1±5.2 | 4.5±4.4 | 9.5±7.8 | <0.001 |
| Serum lactate at 6 hours (mmol/L) | 3.6±4.2 | 3.5±4.1 | 4.9±5.2 | 0.21 |
| Serum lactate clearance (%) | 22.8±21.2 | 22.3±21.1 | 27.4±21.9 | 0.38 |
| Organ support | ||||
| Renal replacement therapyb | (n=116) | (n=98) | (n=18) | |
| Initial serum lactate level (mmol/L) | 7.4±6.2 | 7.0±6.2 | 9.3±5.8 | 0.16 |
| Serum lactate at 6 hours (mmol/L) | 5.5±5.4 | 5.6±5.6 | 4.7±3.7 | 0.69 |
| Serum lactate clearance (%) | 18.5±19.1 | 18.8±19.4 | 16.3±16.4 | 0.75 |
| No renal replacement therapy | (n=661) | (n=588) | (n=73) | |
| Initial serum lactate level (mmol/L) | 4.2±3.5 | 3.9±2.9 | 7.1±6.1 | <0.001 |
| Serum lactate at 6 hours (mmol/L) | 2.9±2.2 | 2.8±1.9 | 4.5±4.2 | <0.001 |
| Serum lactate clearance (%) | 24.9±22.1 | 24.4±21.8 | 29.7±25.7 | 0.19 |
Table 4.
Univariate and multivariate analyses to identify variables independently associated with in-hospital mortality in non-cirrhosis patients
| Clinical characteristics |
Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Age ≥ 65 yr | 1.33 (0.96–1.85) | 0.09 | 1.41 (0.74–2.67) | 0.29 |
| Body mass index ≥21 kg/m2 | 0.63 (0.41–0.95) | 0.03 | 0.44 (0.25–0.77) | <0.01 |
| APACHE II score ≥20 | 2.80 (1.94–4.04) | <0.001 | 2.22 (1.19–4.17) | 0.01 |
| Temperature ≥37.5ºC | 0.62 (0.42–0.92) | 0.02 | 0.65 (0.37–1.13) | 0.13 |
| Respiratory rate ≥25/min | 1.50 (1.08–2.08) | 0.02 | 0.82 (0.46–1.46) | 0.49 |
| Fluid day 1 ≥5,500 ml | 1.34 (0.96–1.80) | 0.06 | 0.73 (0.40–1.34) | 0.31 |
| Maximum vasopressor dose ≥0.2 (µg/kg/min)a | 4.55 (3.21–6.45) | <0.001 | 2.93 (1.54–5.57) | <0.01 |
| Initial serum lactate level ≥4 mmol/L | 1.83 (1.32–2.55) | <0.001 | 1.12 (0.59–2.12) | 0.74 |
| Serum lactate at 6 hours ≥2 mmol/L | 2.62 (1.63–4.22) | <0.001 | 1.15 (0.36–3.67) | 0.81 |
| Serum lactate clearance ≥10% | 0.63 (0.41–0.97) | 0.04 | 1.05 (0.33–3.30) | 0.94 |
| Hypertension | 1.36 (0.98–1.88) | 0.07 | 1.67 (0.89–3.15) | 0.11 |
| Coronary artery disease | 1.58 (1.03–2.41) | 0.04 | 1.03 (0.48–2.25) | 0.93 |
| Pneumonia | 2.33 (1.66–3.27) | <0.001 | 2.26 (1.19–4.28) | 0.01 |
| Urinary tract infection | 0.40 (0.26–0.62) | <0.001 | 0.50 (0.23–1.09) | <0.01 |
| Bacteremia | 1.68 (1.12–2.52) | 0.01 | 2.32 (1.11–4.85) | 0.03 |
| Renal replacement therapy | 4.77 (3.05–7.46) | <0.001 | 2.17 (1.06–4.45) | 0.04 |
| Ventilatory support | 13.11 (7.53–22.84) | <0.001 | 5.70 (2.78–11.67) | <0.001 |
Table 5.
Univariate and multivariate analyses to identify variables independently associated with in-hospital mortality in cirrhosis patients
| Clinical characteristics |
Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| APACHE II score ≥20 | 1.80 (0.74–4.39) | 0.19 | 0.62 (0.14–2.72) | 0.52 |
| Temperature ≥37.0ºC | 0.57 (0.22–1.45) | 0.23 | 0.27 (0.06–1.16) | 0.08 |
| Fluid day 1 ≥5,500 ml | 3.50 (1.43–8.59) | 0.08 | 2.41 (0.61–9.50) | 0.21 |
| Maximum vasopressor dose ≥0.2 µg/kg/mina | 4.59 (1.84–11.45) | <0.01 | 9.52 (1.32–68.86) | 0.03 |
| Initial serum lactate level ≥5 mmol/L | 3.35 (1.30–8.64) | 0.01 | 1.89 (0.34–10.52) | 0.47 |
| Serum lactate at 6 hours ≥5 mmol/L | 4.2 (0.87–20.34) | 0.06 | 0.68 (0.16–2.96) | 0.51 |
| Serum lactate clearance ≥20% | 1.03 (0.23–4.58) | 1.00 | 0.67 (0.12–3.71) | 0.65 |
| Coronary artery disease | 5.00 (0.41–27.41) | 0.04 | 5.20 (0.46–58.76) | 0.18 |
| Chronic kidney disease | 3.71 (1.22–11.61) | 0.02 | 7.40 (0.74–74.36) | 0.09 |
| Receiving adrenaline | 2.39 (1.00–5.73) | 0.05 | 0.60 (0.08–4.49) | 0.62 |
| Receiving dopamine | 6.07 (2.13–17.28) | <0.001 | 0.64 (0.15–2.73) | 0.55 |
| Renal replacement therapy | 0.15 (0.02–1.37) | 0.09 | 0.13 (0.01–1.31) | 0.08 |
| Ventilatory support | 5.00 (1.11–22.44) | 0.04 | 5.16 (1.11–23.97) | 0.04 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download