Abstract
Objective
Surgical clipping of the cerebral aenurysm is considered as a standard therapy with endovascular coil embolization. The surgical clipping is known to be superior to the endovascular coil embolization in terms of recurrent rate. However, a recurrent aneurysm which is initially treated by surgical clipping is difficult to handle. The purpose of this study was to research the management of the recurrent cerebral aneurysm after a surgical clipping and how to overcome them.
Methods
From January 1996 to December 2015, medical records and radiologic findings of 14 patients with recurrent aneurysm after surgical clipping were reviewed retrospectively. Detailed case-by-case analysis was performed based on preoperative, postoperative and follow-up radiologic examinations and operative findings. All clinical variables including age, sex, aneurysm size and location, type and number of applied clips, prognosis, and time to recurrence are evaluated. All patients are classified by causes of the recurrence. Possible risk factors that could contribute to those causes and overcoming ways are comprehensively discussed.
Results
All recurrent aneurysms after surgical clipping were 14 of 2364 (0.5%). Three cases were males and 11 cases were females. Mean age was 52.3. At first treatment, nine cases were ruptured aneurysms, four cases were unruptured aneurysms, and one case was unknown. Locations of recurrent aneurysm were determined; anterior communicating artery (A-com) (n=7), posterior communicating artery (P-com) (n=3), middle cerebral artery (n=2), anterior cerebral artery (n=1) and basilar artery (n=1). As treatment of the recurrence, 11 cases were treated by surgical clipping and three cases were treated by endovascular coil embolization. Three cases of all 14 cases occurred in a month after the initial treatment. Eleven cases occurred after a longer interval, and three of them occurred after 15 years. By analyzing radiographs and operative findings, several main causes of the recurrent cerebral aneurysm were found. One case was incomplete clipping, five cases were clip slippage, and eight cases were fragility of vessel wall near the clip edge.
Conclusion
This study revealed main causes of the recurrent aneurysm and contributing risk factors to be controlled. To manage those risk factors and ultimately prevent the recurrent aneurysm, neurosurgeons have to be careful in the technical aspect during surgery for a complete clipping without a slippage. Even in a perfect surgery, an aneurysm may recur at the clip site due to a hemodynamic change over years. Therefore, all patients must be followed up by imaging for a long period of time.
Since the first description of microsurgical clipping by Dandy2), it has been conventional treatment for cerebral aneurysms. Although endovascular coil embolization increasingly substitutes surgical clipping, the authors of the Barrow Ruptured Aneurysm Trial (BRAT)8) and the Cerebral Aneurysm Rerupture After Treatment (CARAT)3) studies reported the superior durability of surgical clipping to endovascular coil embolization which is associated with higher rates of recurrence and retreatment.
Recurrent cerebral aneurysm after a clipping is rare. Nevertheless, the complete microsurgical clipping of an aneurysm cannot be achieved in all cases. Some authors have reported that the rate of remnant aneurysm varies from 1.6% to 42%1,4,6,10,14–16). However, recurrent cerebral aneurysms were not studied yet enough. So we investigated the possible causes and the risk factors of recurrence after clipping and discussed how to manage them.
This is a retrospective study with review of medical records and radiologic findings of applicable cases. From January 1996 to December 2015 in our institution, 2364 patients were treated by aneurysmal clipping. Among these patients, 14 patients who showed residual or recurrent aneurysm underwent microsurgical clipping or endovascular coil embolization. Preoperative work-up included digital subtraction angiography (DSA), computed tomography angiography (CTA) or magnetic resonance angiography. The following demographic and clinical variables were recorded : age, sex, and time to recurrence after primary treatment. Among the characteristics of the clipped aneurysms, their location, size, and preoperative rupture were assessed. The kinds of the primary clip were also identified.
By detailed evaluation with radiographs and operative findings, main causes of recurrent aneurysm were defined. Then all cases are classified by each cause. Based on collected patient data, clinical experience, and other authors’ literature, the risk factors that could contribute to those main causes were investigated. Then, a comprehensive discussion about the ways of overcoming was done.
Basic characteristics and clinical variables of the patients are recorded in Table 1. Fourteen patients (11 females and 3 males) suited to the criteria. Location of the aneurysm recurrence were anterior communicating artery (n=6, 42.8%), posterior communicating artery (n=3, 21.4%), anterior cerebral artery (n=1, 7.1%), middle cerebral artery (n=2, 14.3%), and basilar artery (n=1, 7.1%). The size of initial aneurysm ranged from 2.4 to 11.2 mm in the greatest diameter (mean, 3.84–6.98 mm). But six cases had no pre-operative images, so their size could not be measured.
The clinical presentation in the first operation was as follows; 10 patients underwent the emergency operation because of the subarachnoid hemorrhage, and four patients received the surgery after accidental detection. The clips used in the primary treatment were either YASARGIL (Aesculap AG, Tuttlingen, Germany) (n=6, 50%) or SUGITA (Mizuho Medical Co., Ltd., Tokyo, Japan) (n=6, 50%). In one case, both of them were used together. In another case, the type of clip was not known.
As described in Table 2, incomplete clipping were showed in CTA images of one patient (case 2). The clipping in five patients (case 6, 7, 9, 10, and 14) mechanically failed by slippage. In the review of the radiologic findings, the rest of the eight patients had neither inappropriate clipping nor clip slippage. The mean time for an aneurysm to recur after an initial treatment was 7.25 years.
This 56-years-old female patient who had undergone an aneurysm clipping operation 15 years ago presented general weakness and was diagnosed with subarachnoid hemorrhage (SAH) by computed tomography (CT). DSA confirmed dog ear formation indicating recurrent aneurysm right beside the previously clipped anterior communicating arterial aneurysm. The patient then underwent a craniotomy for aneurysm clipping. There was a tough adherent connective tissue between the clips, and the aneurysm wall. Although there were torn regions and bleeding occurred, a delicate dissection minimized aneurysm rupture, and unclipping was done. Then, the recurrent aneurysm was completely ligated by cotton-clipping technique. According to the radiographs from the past to the present, the aneurysm was completely clipped and the clip had never been slipped. Operative findings noted that aneurysm had recurred right beside the previously clipped aneurysm after a long period of time. In conclusion, this recurrent aneurysm was caused by a fragility of vessel wall (Fig. 1).
This 76-years-old male patient presenting headache was diagnosed with SAH using CT. DSA confirmed a saccular anterior cerebral arterial aneurysm. The patient underwent craniotomy for aneurysm clipping. Post-operative CTA image showed complete ligation of aneurysm. After a discharge, twenty days after the first operation, the patient visited emergency room with mental deterioration, and he was diagnosed with SAH by CT. DSA confirmed a displacement of the clips from the aneurysm. Contrary to the post-operative CTA image, it suggested that the significant clip slippage. The patient then underwent a surgical revision and the aneurysm was clipped again with an additional clip. Finally, post-operative DSA confirmed the complete ligation and recurrence did not occur during the follow up period. As a result, this recurrent aneurysm was caused by a clip slippage in reference to the radiographs and operative findings (Fig. 2).
Microsurgical clipping of a cerebral aneurysm is superior to endovascular coiling in regard to occlusion rates and rebleeding risk7,10). Nevertheless, our study showed that patients who underwent operation for original aneurysms still carry a risk for recurrence. The incidence of SAH was estimated from 0.0% to 0.03% per year in the general population in numerous studies5,12,13). Several authors reported that the recurrence rate for SAH was more than 10 times higher17).
By careful analysis of each case, at last, the main causes of recurrent aneurysm are defined. The first main cause is incomplete clipping. In case 2, by analyzing the radiologic findings right after the first surgical clipping, small remnant aneurismal necks were found near the clip site. The complete occlusion of an aneurysm by a clip is not always possible. Thereby, numerous experiences and the operator’s ability are the most important issues, so a careful consideration about surgical technique is necessary. Moreover, supplemental methods are required to confirm the completeness of the clipping, and to reduce mistakes on surgical decisions during surgery.
Cases 6, 7, 9, 10, and 14 are found to be caused by a significant clip slippage, which is one of the main causes of recurrence. The radiologic findings after the first surgical clipping demonstrated radiologic complete clipping of aneurysms without remnant aneurismal neck. However, according to the radiographs during recurrent bleeding, the clips were significantly migrated or completely dislocated from the original position. Therefore, in addition to complete clipping, the effective solution to the sustainability of the applied clips also became an important issue.
Although there was no incomplete clipping or clip slippage at the first treatment, recurrent aneurysm occurred in cases 1, 3, 4, 5, 8, 11, 12, and 13. The radiographs did not show any suspect signs of remnant aneurismal neck or clip dislocation. However, recurrent aneurysms did occur. It was concluded that this phenomenon may come from the fragility of the vessel wall near the clip site. Sustained hemodynamic change over years after clipping can increase fragility of vessel wall at clip edge, and eventually aneurysm could recur. These findings indicate that even if the clipping is perfect, recurrent aneurysm still can occur. Moreover, in this study, this was the most common process of recurrent aneurysm. Except case 1, rest of the cases is treated with surgical revision. In this situation, because of a tough adherent connective tissue between the clips and the aneurysm wall, a delicate and careful dissection avoiding aneurysm rupture and unclipping are needed. If a tear and bleeding of an aneurysm develop during dissection, a cotton-clipping technique may be a good choice.
Possible risk factors contributing to main causes above are discussed in following categories : aneurysm-related factors, clip-related factors, surgical technique-related factors and others. Among the clinical variables associated with the aneurysm, the location of the aneurysm was found to be a significant risk factor. By statistical analysis of location, the aneurysms on anterior communicating artery was the most common location (6 of 14, 42.8%).
Papadopoulos et al.9) reported multiple open/close cycle and sustained opening on the clip applier weaken the grip strength of clips. If the operator sustained maximal opening for as little as 10 minutes, the closing force decreased by 20–25%. In addition, repetitive opening and closing such like scissoring action caused weakness further by 12%. Actually, in many institutes, the clips on the tray are sterilized at high temperatures several times until they are used. Repeated sterilization might also weaken the clip power. Some types of clip are not frequently used. Therefore, infrequently used types of clip on the tray undergo sterilization many times. The less frequent the usage of the clip, the higher the possibility of this process. Then, the clip power weakened by this process leads to an incomplete clipping or clip slippage. Therefore, to preserve the grip strength of clips, the operators should keep these in mind during surgery.
The complete clipping is an ultimate goal of treatment. However, as mentioned above, it is not always possible. Sometimes clipping that has been shown to be perfect during surgery reveals remnant neck on post-operative radiographs. But there are several ways to complement those for complete clipping. Some authors perform intraoperative DSA after clipping, and if necessary, they adjust the clips immediately. A real time visualization of the intracranial vasculature such as a videoangiography with indocyanine green or fluorescein can also be greatly helpful for complete clipping11,18). Currently, we usually use the videoangiography in order to confirm complete clipping (Fig. 3). Meanwhile, in addition, electrocauterization of aneurysmal sac during surgery should be avoided, because it may decrease the size of aneurismal sac and possibly leading to clip slippage. Moreover, clipping aneurysm parallel to the parent vessel is known to be ideal for complete clipping. Thus, to keep this point, the operators should utilize clips of various and appropriate shapes.
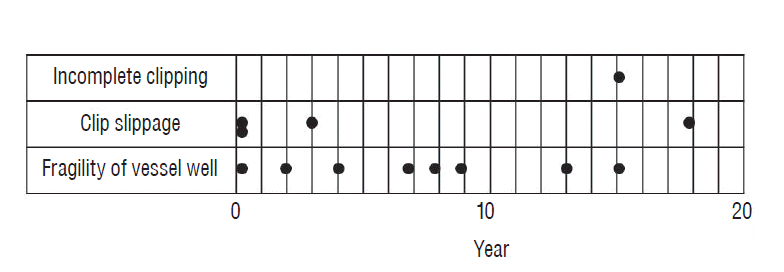
As a result of the analysis of the interval from initial treatment to recurrence, a significant correlation between the interval and main causes was found. Each cause included one case that recurred within a month. The rate of the number of cases that recurred after a longer interval of more than one month for each causes was calculated as follows; incomplete clipping (one of 11, 9%), clip slippage (three of 11, 27.2%), fragility of vessel wall (seven of 11, 63.6%). The longer the intervals, the higher the relationship to the long term of hemodynamic change. The recurrence due to long term of hemodynamic change even occurs in technically perfect surgeries. In this study, there were 4 patients who took more than 10 years to recur. Moreover, it took 18 years from initial treatment in case 6. Therefore, follow up examination must be indicated more than 10 years after surgery even for patients with completely clipped aneurysm (Fig. 4).
The surgical clipping has better recurrence rate rather than endovascular coil embolization. However, a recurrent aneurysm with surgical clipping as an initial treatment has less treatment options than coiled one, because the severe adhesion of the surgical site due to previous surgery makes the surgical revision very difficult. Therefore, neurosurgeons should manage recurrence more carefully in case of treating with surgical clipping rather than endovascular coil embolization.
This study is limited in aspect of that it is retrospective research and has been studied with a small group of subjects by a single surgeon in a single institute. Moreover, insufficient medical records and radiographs, especially outdated cases, couldn’t afford a comparative study between recurrent and non-recurrent aneurysm enough. If we had larger and more qualified group of subjects by multiple institute, we may have discovered better results about relationship between possible risk factors and recurrent aneurysm with more reliable evidence.
Several risk factors of the recurrent aneurysm after clipping were revealed from this study. There are corresponding ways to manage each of them. The most important thing is whether neurosurgeons perform a high-quality clipping during surgery. With appropriate surgical techniques and useful supplemental methods, the neurosurgeons must clip aneurysm completely and ensure that the clip does not slip. However, although the clipping is perfect without any slippage, the recurrence of aneurysm can occur due to a hemodynamic change over years at the clip site. Therefore, all patients who underwent surgical clipping of cerebral aneurysm need to be followed up by imaging for a long period of time.
References
1. Ahn SS, Kim YD. Three-dimensional digital subtraction angiographic evaluation of aneurysm remnants after clip placement. J Korean Neurosurg Soc. 47:185–190. 2010.

2. Dandy WE. Intracranial aneurysm of the internal carotid artery: cured by operation. Ann Surg. 107:654–659. 1938.

3. Elijovich L, Higashida RT, Lawton MT, Duckwiler G, Giannotta S, Johnston SC, et al. Predictors and outcomes of intraprocedural rupture in patients treated for ruptured intracranial aneurysms: the CARAT study. Stroke. 39:1501–1506. 2008.

4. Ihm EH, Hong CK, Shim YS, Jung JY, Joo JY, Park SW. Characteristics and management of residual or slowly recurred intracranial aneurysms. J Korean Neurosurg Soc. 48:330–334. 2010.

5. Inagawa T, Ishikawa S, Aoki H, Ishikawa S, Yoshimoto H. Aneurysmal subarachnoid hemorrhage in izumo city and shimane prefecture of Japan. Incidence Stroke. 19:170–175. 1988.

6. Kang HS, Han MH, Kwon BJ, Jung SI, Oh CW, Han DH, et al. Postoperative 3D angiography in intracranial aneurysms. AJNR Am J Neuroradiol. 25:1463–1469. 2004.
7. Li H, Pan R, Wang H, Rong X, Yin Z, Milgrom DP, et al. Clipping versus coiling for ruptured intracranial aneurysms: a systematic review and meta-analysis. Stroke. 44:29–37. 2013.

8. McDougall CG, Spetzler RF, Zabramski JM, Partovi S, Hills NK, Nakaji P, et al. The barrow ruptured aneurysm trial. J Neurosurg. 116:135–144. 2012.

9. Papadopoulos MC, Apok V, Mitchell FT, Turner DP, Gooding A, Norris J. Endurance of aneurysm clips: mechanical endurance of yasargil and spetzler titanium aneurysm clips. Neurosurgery. 54:966–972. 2004.

10. Rauzzino MJ, Quinn CM, Fisher WS 3rd. Angiography after aneurysm surgery: indications for “selective” angiography. Surg Neurol. 49:32–41. discussion 40–41. 1998.

11. Jabbarli R, Pierscianek D, Wrede K, Dammann P, Schlamann M, Forsting M, et al. Aneurysm remnant after clipping: the risks and consequences. J Neurosurg. 125:1249–1255. 2016.

12. Sacco RL, Wolf PA, Bharucha NE, Meeks SL, Kannel WB, Charette LJ, et al. Subarachnoid and intracerebral hemorrhage: natural history, prognosis, and precursive factors in the Framingham Study. Neurology. 34:847–854. 1984.

13. Sarti C, Tuomilehto J, Salomaa V, Sivenius J, Kaarsalo E, Narva EV, et al. Epidemiology of subarachnoid hemorrhage in Finland from 1983–1985. Stroke. 22:848–853. 1991.

14. Sindou M, Acevedo JC, Turjman F. Aneurysmal remnants after microsurgical clipping: classification and results from a prospective angiographic study (in a consecutive series of 305 operated intracranial aneurysms). Acta Neurochir (Wien). 140:1153–1159. 1998.

15. Thornton J, Bashir Q, Aletich VA, Debrun GM, Ausman JI, Charbel FT. What percentage of surgically clipped intracranial aneurysms have residual necks? Neurosurgery. 46:1294–1300. discussion 1298–1300. 2000.

16. Tsutsumi K, Ueki K, Morita A, Usui M, Kirino T. Risk of aneurysm recurrence in patients with clipped cerebral aneurysms: results of long-term follow-up angiography. Stroke. 32:1191–1194. 2001.

Fig. 1
Case 5. Images providing an example of a recurrent aneurysm due to fragility of vessel wall. This patient with a dog ear formation (white arrow) right beside previous clips (black arrow) demonstrated by the preoperative DSA (A). The patient underwent a craniotomy for aneurysm clipping. There was a tough adherent connective tissue between the clips and aneurysm wall (B). A delicate dissection avoiding aneurysm rupture and unclipping was done. Then, the recurrent aneurysm was completely ligated by cotton-clipping technique (C). The postoperative DSA demonstrated a complete clipping (D). DSA : digital subtraction angiography.
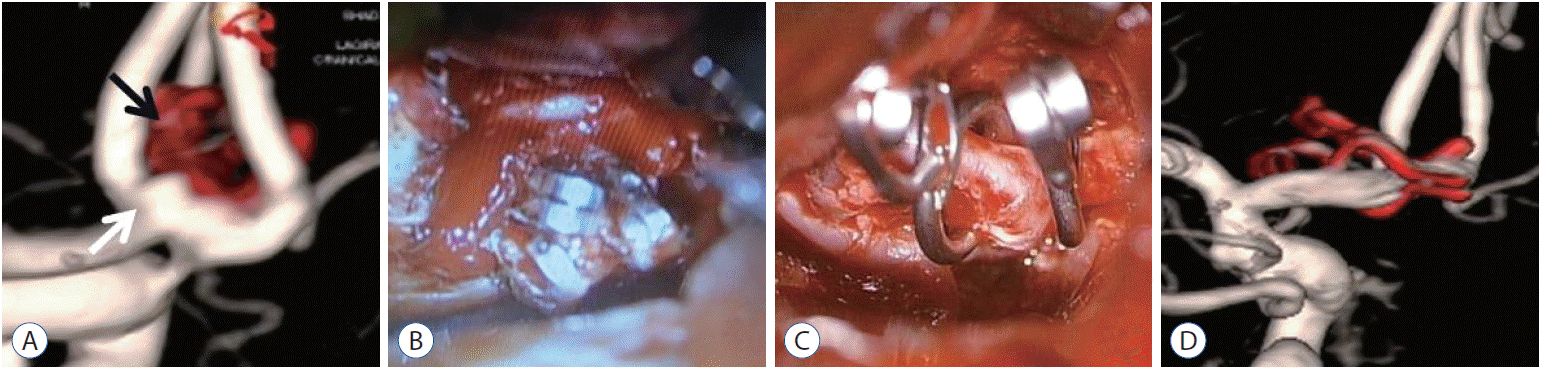
Fig. 2
Case 10. Images providing an example of a recurrent aneurysm due to incomplete clipping. The patient presented with ruptured aneurysm on anterior cerebral artery (white arrow) underwent a surgical clipping (A). Contrary to post-operative computed tomography angiography image after the first clipping (B), the DSA after recurrent subarachnoid hemorrhage demonstrated a displacement of the clips from the aneurysm (C). The patient then underwent surgical revision, and the aneurysm was clipped again with an additional clip. Finally, post-operative DSA confirmed the complete ligation (D) and recurrence did not occur during the follow up period. DSA : digital subtraction angiography.
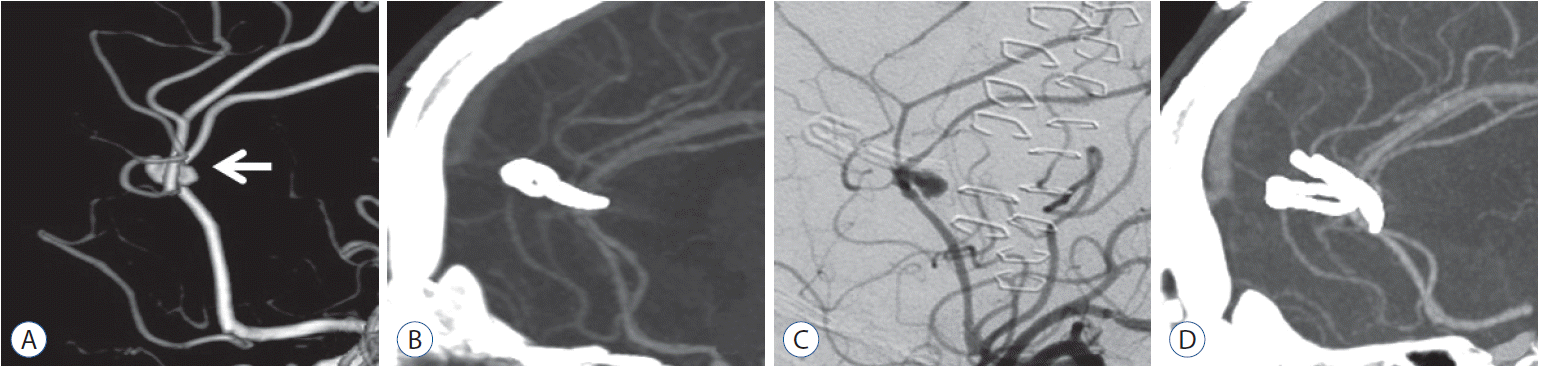
Fig. 3
An example showing usefulness of intraoperative videoangiography. A 63-years-old female patient with a ruptured aneurysm on middle cerebral arterial bifurcation underwent craniotomy and clip ligation (A and B). Following intraoperative videoangiography with indocyanine green showed blood flow that remained inside the aneurysm indicating incomplete obliteration (C, arrowhead). Therefore, an additional clip was immediately applied to the aneurysm. The fluorescein videoangiography was subsequently performed in few minutes and finally, during surgery, the complete obliteration was confirmed (D).
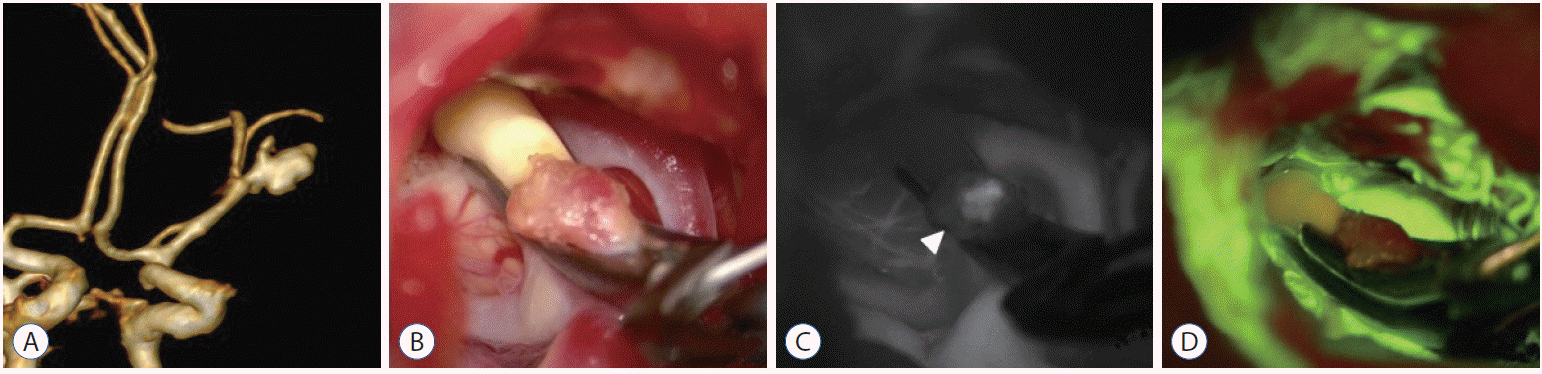
Table 1
Demographics of the patients




 Citation
Citation Print
Print


 XML Download
XML Download