INTRODUCTION
MATERIALS AND METHODS
Study design
Patient data
Premedication protocol and antiplatelet response assay
Procedure
Follow-up
Statistics
RESULTS
Responsive group vs. hypo-responsive group
Table 1
| Hypo-responsive group (PRU≥240, n=10) | Responsive group (PRU<240, n=37) | p-value* | |
|---|---|---|---|
| Clinical | |||
| Age (years) | 59.10±10.71 | 55.27±11.49 | 0.348 |
| Gender (female) | 9 (90) | 22 (59.5) | 0.131 |
| Diabetes | 2 (20) | 4 (10.8) | 0.594 |
| Hypertension | 4 (40) | 14 (37.8) | 1.000 |
| Smoking | 2 (20) | 4 (10.8) | 0.594 |
| Alcoholism | 0 | 0 | - |
| History of stroke | 1 (10) | 4 (10.8) | 1.000 |
| Heart problem | 0 | 2 (5.4) | 1.000 |
| BMI (kg/m2) | 25.86±2.85 | 24.56±3.52 | 0.290 |
|
|
|||
| Laboratory | |||
| Pre-PRU | 271.10±26.52 | 181.27±44.4 | <0.001 |
| Post-PRU | 100.10±79.29 | 64.19±48.85 | 0.227 |
| PRU variability | 171.00±59.30 | 117.08±62.94 | 0.019 |
| Cholesterol (mg/dL) | 151.75±25.20 | 184.21±75.18 | 0.222 |
| TG (mg/dL) | 92.75±44.33 | 157.24±170.06 | 0.155 |
| HDL (mg/dL) | 53.75±12.97 | 47.88±16.66 | 0.165 |
| LDL (mg/dL) | 94.88±21.93 | 103.67±31.31 | 0.459 |
| GFR (mL/min) | 88.40±18.48 | 92.58±15.35 | 0.468 |
| Platelets (103/μL) | 232.90±54.16 | 230.16±57.12 | 0.893 |
|
|
|||
| Medications | |||
| Statin | 5 (50) | 13 (35.1) | 0.473 |
| Metformin | 2 (20) | 2 (5.4) | 0.194 |
| PPI | 0 | 3 (8.1) | 1.000 |
| H2-blocker | 4 (40) | 19 (51.4) | 0.724 |
| SSRIs | 1 (10) | 1 (2.7) | 0.384 |
| β-blocker | 0 | 3 (8.1) | 1.000 |
| CCB | 3 (30) | 11 (29.7) | 1.000 |
| ACEi or ARB | 3 (30) | 10 (27) | 1.000 |
| Diuretics | 1 (10) | 3 (6.1) | 1.000 |
| Nitrate | 0 | 0 | - |
| Warfarin | 1 (10) | 1 (2.7) | 0.384 |
* p-value was determined with the chi-square test or Fisher’s exact test in categorical variables and Student’s t-test or Mann-Whitney test in parametric variables.
PRU: P2Y12 receptor reaction unit, BMI: body mass index, pre-PRU: P2Y12 receptor reaction unit at admission, post-PRU: P2Y12 receptor reaction unit at 1 month after the procedure, PRU variability: pre-PRU–post-PRU, TG: triglyceride, HDL: high density lipoprotein, LDL: low density lipoprotein, GFR: glomerular filtration rate, PPI: proton pump inhibitor, H2-blocker: histamine-2 receptor antagonist, SSRI: selective serotonin reuptake inhibitor, CCB: calcium channel blocker, ACEi: angiotensin-converting enzyme inhibitor, ARB: angiotensin II receptor blocker
PRU variability
Table 2
| Large variation (variation ≥128.55, n=27) | Small variation (variation <128.55, n=20) | Univariate (p-value*) | Multivariate (p-value [OR, 95% CI]) | |
|---|---|---|---|---|
| Clinical | ||||
| Age (years) | 55.70±9.47 | 56.60±3.68 | 0.792 | |
| Gender (female) | 20 (74.7) | 11 (55) | 0.172 | |
| Diabetes | 4 (14.8) | 2 (10) | 1.000 | |
| Hypertension | 14 (51.9) | 4 (20) | 0.026 | 0.353 (4.579, 0.185–113.284) |
| Smoking | 4 (14.8) | 2 (10) | 1.000 | |
| Alcohol | 0 | 0 | - | |
| History of stroke | 3 (11.1) | 2 (10) | 1.000 | |
| Heart problem | 1 (3.7) | 1 (5) | 1.000 | |
| BMI (kg/m2) | 25.01±3.63 | 24.60 ± 3.13 | 0.689 | |
|
|
||||
| Laboratory | ||||
| Pre-PRU | 220.11 ± 35.82 | 173.75±66.01 | 0.008 | 0.005 (1.034, 1.010–1.059) |
| PRU hypo-responder (pre-PRU≥240) | 8 (25.6) | 2 (10) | 0.154 | |
| Cholesterol (mg/dL) | 192.39±85.37 | 162.00±34.44 | 0.193 | |
| TG (mg/dL) | 160.04±200.07 | 125.00±65.49 | 0.703 | |
| HDL (mg/dL) | 51.65±18.47 | 45.67±11.91 | 0.357 | |
| LDL (mg/dL) | 106.09±23.18 | 96.67±36.40 | 0.347 | |
| GFR (mL/min) | 91.95±17.81 | 91.33±13.44 | 0.897 | |
| Platelets (103/μL) | 253.81±48.92 | 199.60±50.21 | 0.001 | 0.004 (1.043, 1.013–1.073) |
|
|
||||
| Medications | ||||
| Statin | 11 (40.7) | 7 (35) | 0.689 | |
| Metformin | 4 (14.8) | 0 | 0.126 | |
| PPI | 2 (7.4) | 1 (5) | 1.000 | |
| H2-blocker | 14 (51.9) | 9 (45) | 0.642 | |
| SSRIs | 2 (7.4) | 0 | 0.500 | |
| β-blocker | 2 (7.4) | 1 (5) | 1.000 | |
| CCB | 10 (37) | 4 (20) | 0.207 | |
| ACEi or ARB | 11 (40.7) | 2 (10) | 0.020 | 0.699 (0.487, 0.013–18.781) |
| Diuretics | 4 (8.5) | 0 | 0.126 | |
| Nitrate | 0 | 0 | - | |
| Warfarin | 1 (3.7) | 1 (5) | 1.000 | |
* p-value was determined with the chi-square test or Fisher’s exact test in categorical variables and Student’s t-test or Mann-Whitney test in parametric variables.
PRU: P2Y12 receptor reaction unit, OR: odds ratio, CI: confidence interval, BMI: body mass index, pre-PRU: P2Y12 receptor reaction unit at admission, TG: triglyceride, HDL: high density lipoprotein, LDL: low density lipoprotein, GFR: glomerular filtration rate, PPI: proton pump inhibitor, H2-blocker: histamine-2 receptor antagonist, SSRI: selective serotonin reuptake inhibitor, CCB: calcium channel blocker, ACEi: angiotensin-converting enzyme inhibitor, ARB: angiotensin II receptor blocker
Thromboembolic infarction, hemorrhagic complication
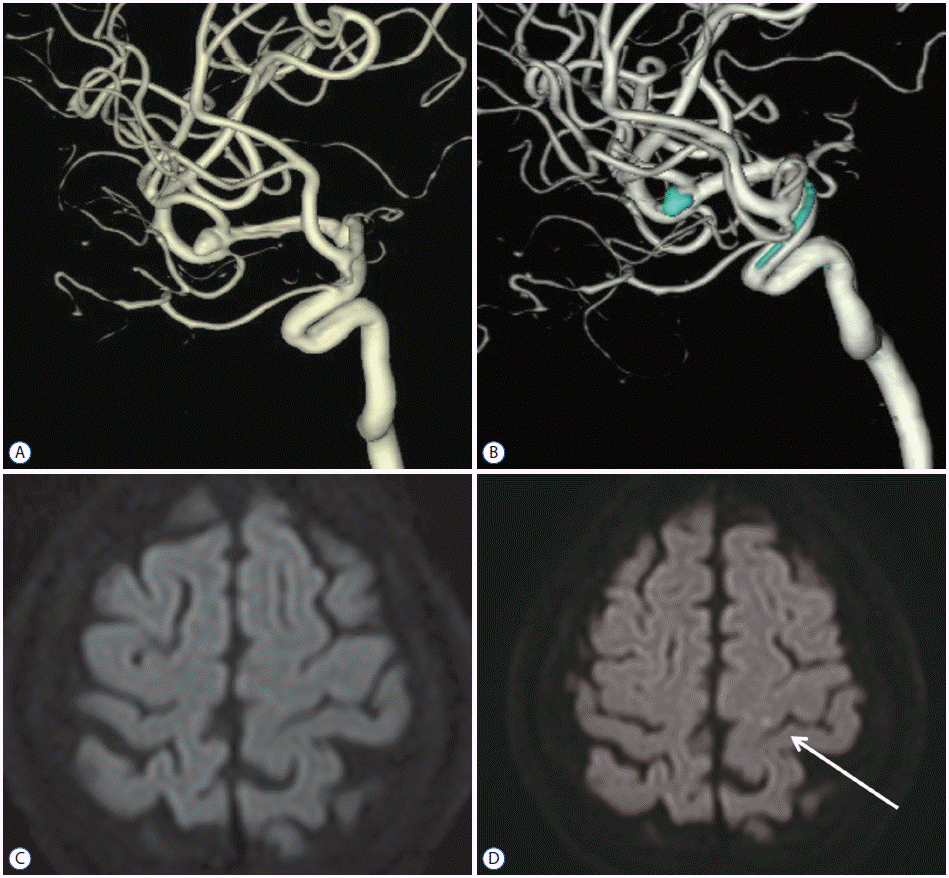 | Fig. 1The patient was a 77-year-old woman who underwent a stent-assisted coil embolization procedure on an anterior communicating artery with an aneurysm 4.4 mm in size. One-year follow-up magnetic resonance imaging revealed a dot-like infarction in the left frontal lobe, but no symptoms. A : Anterior communicating artery aneurysm on angiography before the procedure. B : Anterior communicating artery aneurysm on angiography immediately after the procedure. C : Diffusion weighted imaging at one day after the procedure. D : Diffusion weighted imaging at one year post-operation (the arrow indicates a dot-like infarction). |
Table 3
| Hemorrhagic complication (n=18) | Univariate (p-value*) | Multivariate (p-value† [OR, 95% CI]) | |
|---|---|---|---|
| Clinical | |||
| Age (years) | 52.72±13.03 | 0.109 | |
| Gender (female) | 14 (77.8) | 0.178 | |
| Diabetes | 1 (5.6) | 0.384 | |
| Hypertension | 5 (27.8) | 0.242 | |
| Smoking | 1 (5.6) | 0.384 | |
| Alcohol | 0 | - | |
| History of stroke | 2 (11.1) | 1.000 | |
| Heart problem | 0 | 0.517 | |
| BMI (kg/m2) | 23.84±4.13 | 0.114 | |
|
|
|||
| Laboratory | |||
| Pre-PRU | 172.67±43.53 | 0.002 | 0.033 (0.983, 0.968–0.999) |
| Post-PRU | 55.72±45.01 | 0.212 | |
| PRU variability | 116.94±67.18 | 0.450 | |
| Cholesterol (mg/dL) | 179.27±31.49 | 0.133 | |
| TG (mg/dL) | 122.13±52.65 | 0.685 | |
| HDL (mg/dL) | 49.67±20.80 | 0.507 | |
| LDL (mg/dL) | 112.960±28.30 | 0.081 | 0.178 (1.020, 0.991–1.049) |
| GFR (mL/min) | 96.78±15.95 | 0.085 | 0.788 (0.993, 0.940–1.048) |
| Platelets (103/μL) | 239.94±56.94 | 0.335 | |
|
|
|||
| Medications | |||
| Statin | 3 (16.7) | 0.016 | 0.054 (0.152, 0.022–1.032) |
| Metformin | 0 | 0.283 | |
| PPI | 2 (11.1) | 0.549 | |
| H2-blocker | 11 (61.1) | 0.188 | |
| SSRIs | 1 (5.6) | 1.000 | |
| β-blocker | 1 (5.6) | 1.000 | |
| CCB | 4 (22.2) | 0.372 | |
| ACEi or ARB | 4 (22.2) | 0.739 | |
| Diuretics | 2 (11.1) | 0.631 | |
| Nitrate | 0 | - | |
| Warfarin | 0 | 0.517 | |
* p-value was determined with the chi-square test or Fisher’s exact test in categorical variables and Student’s t-test or Mann-Whitney test in parametric variables.
OR: odds ratio, CI: confidence interval, BMI: body mass index, pre-PRU: P2Y12 receptor reaction unit at admission, post-PRU: P2Y12 receptor reaction unit at 1 month after the procedure, PRU: P2Y12 receptor reaction unit, PRU variability: pre-PRU–post-PRU, TG: triglyceride, HDL: high density lipoprotein, LDL: low density lipoprotein, GFR: glomerular filtration rate, PPI: proton pump inhibitor, H2-blocker: histamine-2 receptor antagonist, SSRI: selective serotonin reuptake inhibitor, CCB: calcium channel blocker, ACEi: angiotensin-converting enzyme inhibitor, ARB: angiotensin II receptor blocker
DISCUSSION
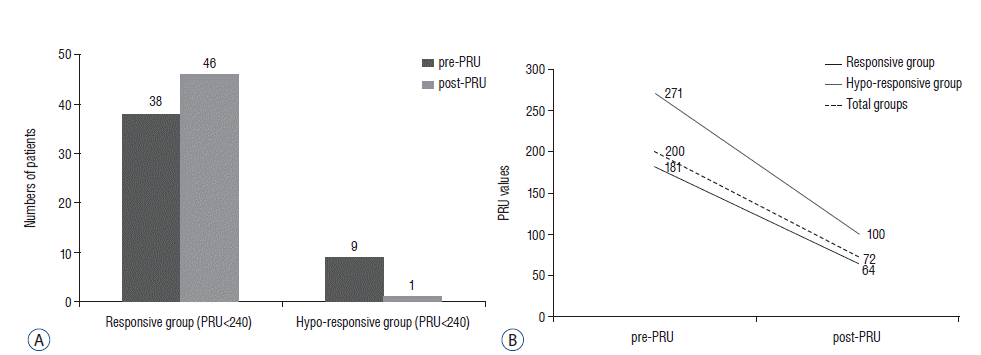 | Fig. 2Clopidogrel response variability in unruptured intracranial aneurysm patients treated with stent assisted coil embolization from initial response to one month after the medication. A : Responsive group vs. hypo-responsive group. B : The variation of PRU from initial response to one month after the medication. PRU : P2Y12 reaction unit. |




 Citation
Citation Print
Print


 XML Download
XML Download