INTRODUCTION
Spondylitis refers to osteomyelitis that arises in the spine. In many cases, spondylitis patients respond poorly to antimicrobial chemotherapy, and even if they do respond to treatment, complete remission takes a long time
20). Therefore, early initiation of chemotherapy is very important to achieve better results and to reduce the neurological sequelae and socioeconomic burden. In many cases, the causative microorganism cannot be isolated. Having a general idea of the most likely causative microorganism is important for empirical chemotherapy of spondylitis. If the patient does not show any definite source of infection such as a preceding infection, history of surgery, prosthesis insertion, or trauma, the spondylitis is defined as spontaneous spondylitis.
Historically, in spontaneous spondylitis, the first step is to differentiate between pyogenic and tuberculous spondylitis. Many authors have studied the differences between pyogenic and tuberculous spondylitis
3,
5,
16,
24). South Korea had many tuberculosis patients and public health worsened after the Korean War. However, recently, the prevalence of tuberculosis in Korea has markedly decreased because of vaccination and adequate chemotherapy
12,
18). The microorganisms that affect the spine are continuously changing
23). Several antibiotic-resistant microorganisms have arisen, and new antibiotics have been developed to address this problem.
We hypothesize that the manifestations of recent spontaneous spondylitis cases would differ from those reported in the past and believe that updated clinical research on this subject is warranted. Here, we present our retrospective study of patients with spontaneous spondylitis during the last 6 years.
Go to :

RESULTS
A total of 69 patients (39 males and 30 females) with a mean age of 60.0 (8–83) years were included. The characteristics of pyogenic and tuberculous spondylitis at the time of diagnosis are listed in
Table 1. The mean age of patients was 60.6 (22–83) and 57.0 (8–83) years in the pyogenic and tuberculous groups, respectively. The pyogenic group showed male predominance (32 males and 19 females), whereas the tuberculous group showed female predominance (7 males and 11 famales). Regarding the medical history, in the pyogenic group, the most frequent underlying diseases and conditions were diabetes, chronic renal failure, liver cirrhosis, steroid use, and alcohol abuse. Particularly, five patients with chronic renal failure were identified in the pyogenic group, while none was identified in the tuberculous group.
Table 1
Characteristics of patients with spontaneous spondylitis
|
Pyogenic |
Tuberculous |
p-value |
|
Total |
51 |
18 |
|
|
|
Male |
32 (62.7) |
7 (38.9) |
0.101 |
|
|
Mean age (years) |
60.6 (22–83) |
57.0 (8–83) |
0.440 |
|
≥60 |
28 (54.9) |
9 (50.0) |
|
|
<60 |
23 (45.1) |
9 (50.0) |
|
|
|
Isolation of microorganism |
27 (52.9) |
14 (77.8) |
0.065 |
|
|
Medical history |
13 (25.5) |
4 (22.2) |
0.782 |
|
Diabetes |
9 (17.6) |
3 (16.7) |
1.000 |
|
Chronic renal failure |
5 (9.8) |
0 (0.0) |
0.316 |
|
Cancer |
1 (2.0) |
1 (5.6) |
1.000 |
|
Liver cirrhosis |
1 (2.0) |
0 (0.0) |
1.000 |
|
Steroid use |
1 (2.0) |
0 (0.0) |
1.000 |
|
Alcohol use |
1 (2.0) |
0 (0.0) |
1.000 |
|
|
Inflammatory marker |
|
Peak ESR (mm/h) |
81.5 (22–121) |
75.6 (26–119) |
0.442 |
|
ESR (≥100 mm/h) |
16 (31.4) |
5 (27.8) |
0.880 |
|
Peak CRP (mg/dL) |
14.08 (0.06–38.00) |
8.50 (0.75–24.00) |
0.009*
|
|
CRP (≥10 mg/dL) |
27 (52.9) |
6 (33.3) |
0.152 |
|
Peak WBCs (×103/μL) |
12.77 |
9.23 |
0.002*
|
|
Neutrophils (%) |
81.1 |
75.1 |
0.132 |
|
|
Length of hospitalization (days) |
56.5 (13–202) |
41.2 (8–192) |
0.001*
|
|
|
Combined infection |
11 (21.6) |
0 (0.0) |
<0.001*
|
|
Sepsis |
6 (11.8) |
0 (0.0) |
|
|
Pneumonia |
3 (5.9) |
0 (0.0) |
|
|
Urinary tract infection |
2 (3.9) |
0 (0.0) |
|
|
|
Surgery |
29 (56.9) |
10 (55.6) |
0.923 |
|
Abscess removal |
26 (51.0) |
5 (27.8) |
0.089 |
|
Stabilization |
16 (31.4) |
8 (44.4) |
0.317 |
|
|
Mortality |
4 (7.8) |
0 (0.0) |
<0.001*
|

Regarding laboratory data, the mean values of inflammatory markers were higher in the pyogenic group than in the tuberculous group. The peak ESR was 81.5 mm/h and 75.6 mm/h in the pyogenic and tuberculous groups, respectively. The peak CRP level was significantly higher in the pyogenic group than in the tuberculous group (14.08 mg/dL and 8.50 mg/dL, respectively, p=0.009). The percentage of patients with high ESR (>100 mm/h) and high CRP level (>10 mg/dL) was slightly higher in the pyogenic group (p>0.05). The peak WBC counts were significantly higher in the pyogenic group than in the tuberculous group (12.77×103/μL and 9.23×103/μL, respectively, p=0.002). However, the proportion of neutrophils was not different between the two groups (81.1% and 75.1%, respectively, p=0.132).
The mean length of hospitalization was 15.3 days longer in the pyogenic group (p=0.001). Twenty-nine and 10 patients in the pyogenic and tuberculous groups, respectively, underwent surgical treatment. Abscess removal was more common in the pyogenic group, whereas stabilization was more common in the tuberculous group (p>0.05). Combined infectious diseases such as pneumonia, urinary tract infection, and sepsis were identified only in the pyogenic group. Four mortality cases were found only in the pyogenic group (p<0.001).
The comparison of radiographic parameters is shown in
Table 2. The average number of affected vertebral bodies was 2.53 and 2.18 in the pyogenic and tuberculous groups, respectively (
p=0.256). The most frequently affected vertebra was the lumbar vertebra in both groups, followed by the thoracolumbar (T11–L1) vertebra in the pyogenic group and the thoracic vertebra in the tuberculous group; however, there were no significant between-group differences (
Table 2). The proportion of patients with vertebral body collapse was significantly higher in the tuberculous group than in the pyogenic group (66.7% and 15.7%, respectively,
p<0.001). The proportion of patients with disc sparing on MRI scans was significantly higher in the tuberculous group than in the pyogenic group (50% and 23.5%, respectively,
p=0.044). Epidural abscess and psoas muscle abscess were more common in the pyogenic group, but the difference did not reach statistical significance. Regarding the measurement of the Cobb angle in the affected vertebral bodies on simple radiographs, the difference between the initial and final Cobb angle was 0.11° in the pyogenic group and 1.46° in the tuberculous group, respectively, and was not significantly different.
Table 2
Radiologic parameters of spontaneous spondylitis
|
Pyogenic |
Tuberculous |
p-value |
|
Number of affected VB |
2.53 (1–9) |
2.18 (1–3) |
0.256 |
|
1 |
1 (2.0) |
1 (5.6) |
|
|
2 |
33 (64.7) |
13 (72.2) |
|
|
3 |
13 (25.5) |
4 (22.2) |
|
|
Over 4 |
4 (7.8) |
0 (0.0) |
|
|
|
Location of affected VB |
|
|
0.160 |
|
Cervical |
2 (3.9) |
1 (5.6) |
|
|
Thoracic (T1–T10) |
6 (11.8) |
6 (33.3) |
|
|
Thoracolumbar (T11–L1) |
13 (25.5) |
4 (22.2) |
|
|
Lumbar |
30 (58.5) |
7 (38.9) |
|
|
|
MRI findings |
|
|
|
|
VB collapse |
8 (15.7) |
12 (66.7) |
<0.001*
|
|
Disc sparing |
12 (23.5) |
9 (50.0) |
0.044*
|
|
Epidural abscess |
28 (54.9) |
6 (33.3) |
0.171 |
|
Psoas muscle abscess |
41 (80.4) |
10 (55.6) |
0.060 |
|
|
Cobb angle on radiograph (degrees) |
|
|
|
|
Initial |
14.1 (0.8–48.6) |
14.9 (0.5–35.3) |
0.636 |
|
Final |
14.2 (0.2–38.5) |
16.3 (1.8–39.3) |
0.634 |
|
Alteration |
0.11 |
1.46 |
0.498 |

The causative organism was isolated in 27 (52.9%) and 14 (77.8%) patients in the pyogenic and tuberculous groups, respectively (
Table 1). The isolated organisms are listed in
Table 3. The most common microorganism in the pyogenic group was
Staphylococcus aureus (
S. aureus).
S. aureus was identified in 12 patients (methicillin-sensitive
S. aureus [MSSA] in 8 patients and methicillin-resistant
S. aureus [MRSA] in 4 patients). Twenty-four patients suspected to have pyogenic spondylitis were treated with empirical antibiotics. Eleven (45.8%) patients were treated with vancomycin or teicoplanin, 10 (41.7%) patients were treated with first-generation cephalosporins, and the remaining 3 patients were treated with other cephalosporins and metronidazole. Among the 14 proven tuberculous spondylitis patients, the causative microorganism was isolated using bone biopsy in 11 patients and using sputum culture or aspiration of the psoas abscess in the remaining 3 patients. Four patients with negative culture were treated with tuberculosis medications after consultation with the division of infectious diseases. Three of them showed MRI findings that were highly suggestive of tuberculous spondylitis and yielded positive results on tuberculin test or had a history of pulmonary tuberculosis. The one remaining patient was empirically diagnosed with tuberculosis after the failure of antibiotics treatment for pyogenic spondylitis.
Table 3
Isolated organisms of spontaneous spondylitis
|
Microbiology |
Value |
|
Pyogenic spondylitis |
51 |
|
Staphylococcus aureus
|
12 (23.5) |
|
MSSA |
8 (15.7) |
|
MRSA |
4 (7.8) |
|
Escherichia coli
|
4 (7.8) |
|
Coagulase-negative Staphylococcus
|
3 (5.9) |
|
Enterococcus
|
2 (3.9) |
|
Streptococcus spp.
|
2 (3.9) |
|
Pseudomonas aeruginosa
|
1 (2.0) |
|
Acinetobacter
|
1 (2.0) |
|
Enterobacteriaceae
|
1 (2.0) |
|
Stenotrophomonas maltophilia
|
1 (2.0) |
|
Negative |
24 (47.1) |
|
|
Tuberculous spondylitis (Mycobacterium tuberculosis) |
18 |
|
Proven using bone biopsy |
11 (61.1) |
|
Proven using sputum culture |
2 (11.1) |
|
Proven using abscess aspiration |
1 (5.6) |
|
Negative |
4 (22.2) |

Go to :

DISCUSSION
Differences between pyogenic and tuberculous spondylitis in terms of clinical manifestations and radiologic findings were found in the present study. The prevalence of tuberculosis in South Korea has decreased in the last 30 years owing to improvements in nutrition and compliance, the National vaccination program, and the development of chemotherapies
12,
18). However, the prevalence is still higher than that in other developed countries
18). Therefore, Korean spine clinicians always consider the possibility of tuberculous spondylitis in clinical practice. The present retrospective review suggests that the prevalence of tuberculous spondylitis is decreasing. Pyogenic spondylitis can arise from hematogenous spread of microorganisms from the skin, respiratory tract, genitourinary tract, gastrointestinal tract, and oral cavity
4). Once the patient develops spondylitis, the elimination of the infection takes a very long time and can be fatal, so the proper choice of antibiotics is important. According to the 2015 Infectious Diseases Society of America (IDSA) guideline for spondylitis, unless the patient’s condition is deteriorating rapidly, it is better to obtain a microbiological diagnosis before starting empirical chemotherapy
2). Isolation of the virulent microorganism, using either bone biopsy or blood culture samples, is the most important step in the treatment of spondylitis
19,
20). In spondylitis cases, computed tomography (CT)-guided bone biopsy is the diagnostic procedure of choice
20). In our department, CT-guided bone biopsy and blood culture were attempted for every patient with spondylitis. In patients with a paraspinal abscess pocket large enough to be aspirated, as shown by MRI, aspiration of the abscess for microbiologic diagnosis was also performed.
According to previous guidelines, chemotherapy should be maintained for 6 weeks for most bacterial spondylitis cases and for 3 months for
Brucella-related spondylitis
2). Though the criteria for the cessation of antibiotics for pyogenic spondylitis vary in each clinic, most studies suggest basing the decision on the improvement of pain and normalization of inflammatory markers
7). In our series, intravenous antibiotics were administered for at least 6 weeks in most patients. If the inflammatory marker levels were elevated or not changed after 4 weeks of chemotherapy, the antibiotics were changed and therapy was maintained until the CRP level was normalized. Forty eight of the 51 patients with pyogenic spondylitis received chemotherapy for 6 to 9 weeks. One patient with microbiologically negative results recovered after treatment for 3 months. There were two mortality cases due to septic shock; one of them was a 61-year-old woman in whom
Streptococcus gallolyticus subspecies
pasteurianus was isolated and who died at 4 weeks of treatment. The other patient was a 79-year-old man with
Stenotrophomonas maltophilia who died after 6 months of treatment.
S. aureus is known to be the most common microorganism associated with any type of osteomyelitis
20). MRSA has risen as an important pathogen also in community-acquired osteomyelitis
22). In the present study, four patients with spontaneous spondylitis were found to be infected with MRSA (
Table 3). This trend is essential when considering empirical chemotherapy. Five patients were transferred to our hospital because spondylitis could not be controlled in spite of chemotherapy for 1 or 2 weeks at other hospitals. All of them were treated with vancomycin or teicoplanin at our hospital for another 6 weeks. In our series, delayed recurrence of spondylitis did not occur after adequate chemotherapy was administered at our hospital.
The ESR and CRP level are deemed useful indicators by many clinicians in understanding infectious conditions including spondylitis. Zilkens et al.
27) suggested that among inflammation markers, the ESR shows a high sensitivity, while the CRP level demonstrates good diagnostic specificity for detecting spondylodiscitis. Previous studies have noted a higher CRP level and ESR in pyogenic spondylodiscitis than in tuberculous spondylodiscitis
13,
17,
26). Lew and Waldvogel
20) suggested assessing the efficacy of antibiotics using CRP levels because the white blood cell count is not reliable, and the CRP level changes more rapidly than the ESR. In the present study, the mean CRP level was significantly higher in the pyogenic group, whereas the ESR did not show a significant difference between groups.
MRI is a useful tool for the diagnosis of spine infection. Spondylitis can be diagnosed in the early stage by endplate edema where T1 images show decreased signal intensity and T2 images show increased intensity
1,
15) (
Figs. 1 and
2). MRI is also a reliable method for assessing the epidural space and the spinal cord
25) with over 90% sensitivity, specificity, and accuracy
1). Owing to the absence of proteolytic enzymes of
Mycobacterium tuberculosis (
M. tuberculosis) and avascular characteristics of the disc, the disc space is usually preserved in tuberculous spondylitis until its nutritional support is affected
8,
15). Previous studies have shown that tuberculous spine infection frequently spares disc involvement in MRI scans
3,
15). However, in our series, the intervertebral disc was not affected in 12 patients in the pyogenic group, and in 11 of these 12 patients, multiple vertebral bodies were involved. While half of the patients in the tuberculous group showed disc sparing, the other half showed disc involvement. Therefore, we suggest that although the skipping pattern strongly suggests tuberculous infection, it cannot be a definite diagnosis.
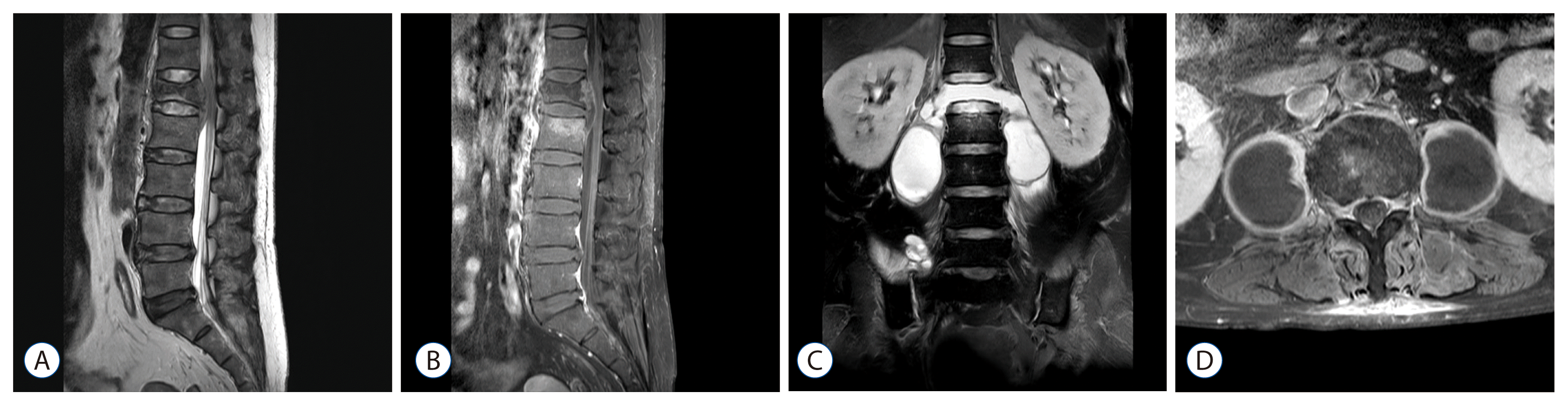 | Fig. 1Representative magnetic resonance imaging (MRI) scans of patients with methicillin resistant Staphylococcus aureus (MRSA) spondylitis. On the initial MRI scan of a 60-year-old woman who was diagnosed as having MRSA spondylitis, the L1 vertebral body was hypointense on a T2 weighted image and showed a bursting fracture with an epidural abscess compressing the conus medullaris (A). On a T1 image with contrast enhancement, the posterior wall of the L1 vertebra and upper endplate of the L2 vertebra showed strong enhancement, and the epidural abscess showed a rim-enhancing pattern (B). Despite abscess removal and vancomycin use, the follow-up MRI scan reveals that the L1 vertebral body had totally disappeared after 2 weeks, communicating with the paraspinal abscesses on both sides (C). Two 4.5×4.0 cm sized abscess pockets were occupying the whole psoas muscles (D), which were removed by image-guided percutaneous drain insertion. 
|
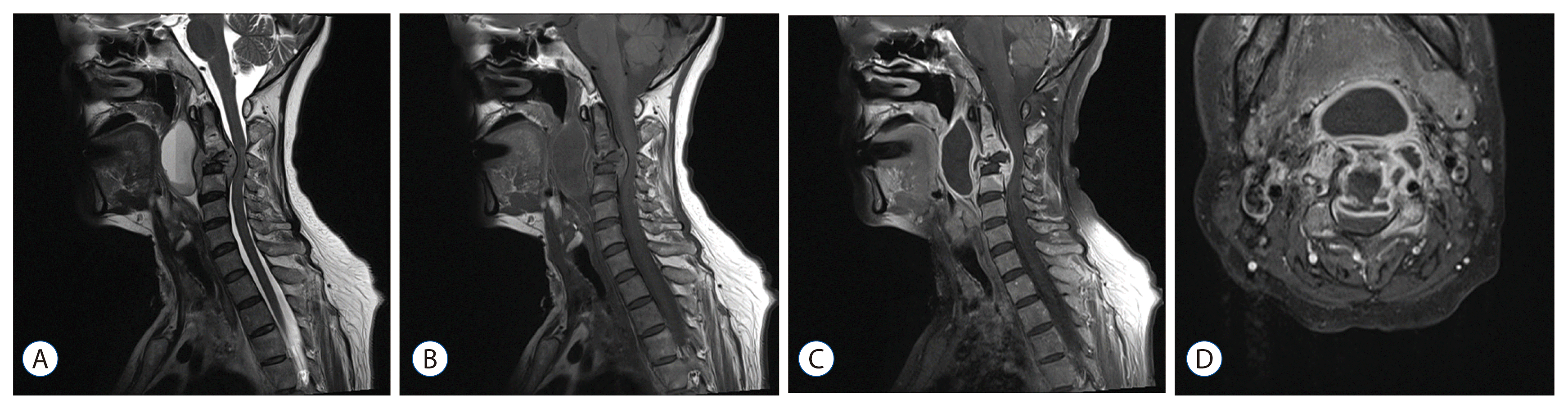 | Fig. 2Representative magnetic resonance images of tuberculous spondylitis. A 57-year old woman presented with hoarseness. A : A T2 sagittal image showed a destructive lesion at the C3 vertebral body compressing the spinal cord and a huge cystic lesion at the retropharyngeal space. Both the destroyed C3 vertebra and cyst were hypointense on T1 (B) and showed strong rim enhancement, whereas the disc spaces were relatively spared (C). On the axial image, the rim enhancing lesion of the vertebral body, epidural, and retropharyngeal space were communicating and severely encroaching the spinal canal (D). 
|
The pattern on contrast-enhanced MRI is very important in the diagnosis of spondylitis
3,
22). Tuberculous spondylitis tends to have a heterogeneous enhancement pattern of the vertebral body, whereas pyogenic spondylitis usually shows a homogenous pattern
22). Moreover, an abscess with rimmed enhancing walls can be visualized on MRI with contrast agents
25). In our series, 47 of the 51 patients showed contrast enhancement of the vertebral bodies at the end of antibiotic therapy even when the inflammatory markers were normalized. This finding suggests that the completion of chemotherapy does not require elimination of contrast enhancement on MRI.
On MRI, a psoas muscle abscess was observed in 80.4% (41/51) and 55.5% (10/18) patients in the pyogenic and tuberculous groups, respectively (
Table 2). If the paraspinal abscess is large, image-guided percutaneous abscess drainage is important to control infection and provide a microbiologic diagnosis. If the spondylitis is combined with an epidural abscess and the patient presents any neurologic symptoms, the patient should undergo surgical removal of the abscess and decompression of the neural structures. In the present study, 54.9% (28/51) and 33.3% (6/18) patients in the pyogenic and tuberculous groups, respectively, had an epidural abscess and most underwent surgery for evacuation of the epidural abscess. Sometimes, the decompressive surgery provides an important clue regarding the etiology of the spondylitis, such as caseating granuloma formation that suggests tuberculosis or purulent discharge, which in turn indicates bacterial infection.
Some spondylitis cases require spinal instrumentation because of the instability caused by the extensive bone destruction. The risk of biofilm formation exists, and the biofilm can make the eradication of the infection difficult
6,
9,
10). However, studies suggest that if the proper antibiotic is administered in the early stage of biofilm infection, it can be cured without removal of the medical device
10). In 2016, in the IDSA guideline for tuberculosis, Nahid et al.
21) suggested chemotherapy with rifampin for 6 to 9 months for tuberculous spondylitis and for 12 months in patients with instrumentation. In our series, 31.4% (16/51) and 44.4% (8/18) patients in the pyogenic and tuberculous groups, respectively, underwent instrumentation (
Table 1). We suggest that if a patient shows spinal instability or if a patient is diagnosed with a bacterial infection, instrumentation should be postponed until after the infection is controlled to prevent biofilm formation. However, if the microorganism is not considered a biofilm-forming species such as
M. tuberculosis11), the stabilization surgery does not need to be postponed.
In our review, vertebral body destruction in spondylitis was strongly associated with the type of organism. Two-thirds (12/18) of the patients showed destructive lesion in the tuberculous group (
Fig. 2). Among the 8 cases with vertebral body destruction in the pyogenic group, the causative microorganism was
S. aureus in 4 cases (2 MRSA and 2 MSSA) (
Fig. 1). Though vertebral body destruction was more common in the tuberculous group, mortality was observed only in the pyogenic group. We suggest that tuberculous spondylitis and pyogenic spondylitis caused by
S. aureus require more frequent radiologic follow-up to monitor the development of deformities.
In spontaneous spondylitis, both pyogenic and tuberculous spondylitis usually result from hematogenous spread
4,
8,
20). Thoracolumbar vertebrae have comparatively richer blood supply, and this area is more frequently involved in spondylitis cases. Because the segmental artery supplies two contiguous vertebral bodies, the hematogenous spread of spondylitis can affect multiple vertebral bodies even at the early stage of the disease
4). In 2010, Kim et al.
17) demonstrated that tuberculous spondylitis is associated with the involvement of over three levels. However, in our study, there was no difference in the number of affected levels. We suspect that with improvements in the early diagnosis of tuberculosis, the number of patients with extensive disease has decreased.
This study has some drawbacks. This is a retrospective study, and the data were collected from patients in a single neurosurgical department. The number of patients in the tuberculous spondylitis group was small. The number of tuberculosis cases in the general population has decreased; therefore, we rarely encounter tuberculosis cases in our department. Although patients with postoperative infection were not included in the present study (patients who underwent spine surgery or prosthetic device insertion within the last 5 years were excluded), the majority of spondylitis cases in our department were caused by postoperative infection. In contrast, the proportion of patients with severe predisposing medical conditions may have been underestimated; thus, the possibility of bias in this regard cannot be denied.
Go to :







 Citation
Citation Print
Print


 XML Download
XML Download