Abstract
Objective
Massive intracerebral hemorrhage (ICH) and major infarction (MI) are devastating cerebral vascular diseases. Decompression craniectomy (DC) is a common treatment approach for these diseases and acceptable clinical results have been reported. Author experienced the postoperative intracranaial pressure (ICP) trend is somewhat different between the ICH and MI patients. In this study, we compare the ICP trend following DC and evaluate the clinical significance.
Methods
One hundred forty-three patients who underwent DC following massive ICH (81 cases) or MI (62 cases) were analyzed retrospectively. The mean age was 56.3±14.3 (median=57, male : female=89 : 54). DC was applied using consistent criteria in both diseases patients; Glasgow coma scale (GCS) score less than 8 and a midline shift more than 6 mm on brain computed tomography. In all patients, ventricular puncture was done before the DC and ICP trends were monitored during and after the surgery. Outcome comparisons included the ictus to operation time (OP-time), postoperative ICP trend, favorable outcomes and mortality.
Results
Initial GCS (p=0.364) and initial ventricular ICP (p=0.783) were similar among the ICH and MI patients. The postoperative ICP of ICH patients were drop rapidly and maintained within physiological range if greater than 80% of the hematoma was removed. While in MI patients, the postoperative ICP were not drop rapidly and maintained above the physiologic range (MI=18.8 vs. ICH=13.6 mmHg, p=0.000). The OP-times were faster in ICH patients (ICH=7.3 vs. MI=40.9 hours, p=0.000) and the mortality rate was higher in MI patients (MI=37.1% vs. ICH=17.3%, p=0.007).
Conclusion
The results of this study suggest that if greater than 80% of the hematoma was removed in ICH patients, the postoperative ICP rarely over the physiologic range. But in MI patients, the postoperative ICP was above the physiologic range for several days after the DC. Authors propose that DC is no need for the massive ICH patient if a significant portion of their hematoma is removed. But DC might be essential to improve the MI patients’ outcome and timely treatment decision.
Massive intracerebral hemorrhage (ICH) with hematoma’s larger than 80 mL, and major infarction (MI) with more than 2/3 of middle cerebral artery (MCA) territory or MCA or intracarotid artery occlusion, are devastating cerebral vascular accident12,16). The management of massive brain swelling caused by ICH or MI, remains an unsolved problem in the field of neurology. Because elevated intracranaial pressure (ICP) is a major predictor of secondary brain injury and poor outcome in these patients, it seems logical to do maximum effort toward preventing intracranial hypertension is warranted1,3,4,12,16,19,21,22,27,29,31,34,38–40). Many reports focused on the treatment of extreme cases of massive ICH or MI, using decompression craniectomy (DC), show that it is effective in managing the uncontrolled increased ICP and acceptable clinical outcome has been reported1,3,11,16,19,21,23,27,29,34,38–40).
Since the brain is encased in the unyielding vault of the skull, the increased volume of brain and blood due to cerebrovascular accident causes results in increased ICP. DC remove the large areas of the skull bone, and convert the intracranial space as a ‘closed box’ with a finite volume into an ‘open box’ and thereby increases compliance that will shift to the right of the pressure-volume curve4,9,18,22–25,34,36,38,42). These pathophysiological processes result in effective lowering of the increased ICP, improvement in cerebral perfusion and prevention of secondary brain damage.
Many previous studies have reported on the benefits and limitations of DC in various neurosurgical diseases, such as ICH, MI, subarachnoid hemorrhage (SAH), traumatic brain injury (TBI) and others. Postoperative monitoring of ICP in TBI is recommend for the timely and proper additional treatment decision21,42). In authors’ institutes DC surgery performed frequently on various neurosurgical diseases, and reported treatment effect of DC on various neurosurgical diseases22,42). In this study, authors would like to compare the postoperative ICP trend in massive ICH and MI following DC surgery and clinical significance of postoperative ICP monitoring in these patients.
The treatment protocol (Fig. 1) was approved by our institutional review board. All patients or their representatives provided informed written consent for surgical management. This study is a retrospective observatory data analysis, comparing the time to operation-time (OP-time), postoperative ICP trend, and clinical outcomes between the ICH and MI patients with DC.
One hundred forty-three patients who underwent DC following massive ICH (81 cases) and MI (62 cases) were analyzed retrospectively (Table 1). Mean patients’ age was 56.3±14.3 (median=57) and male to female ratio was 89 : 54. Unilateral and bilateral DC was applied in 97 cases and 46 cases, and repeated surgery was done 7 cases for removing the remained ICH (with 4 ending in death and the remaining 3 considered unfavorable). In MI patients, if the postoperative ICP increased high, additional medical treatment and metabolic therapies (hypothermia or coma therapy) applied rather than reoperation. But in 6 cases of MI patients, additional DC of the opposite side after initial DC was done, in these 6 cases data were not included in this analysis, because their operation indication were not constant and hard to analyze clinical result (with 3 ending in death and the remaining 3 considered unfavorable).
DC was indicated in cerebrovascular patients with Glasgow coma scale (GCS) score less than 8 due to an intracerebral hemorrhage with a volume of over 80 mL, and a midline shift greater than 6 mm due to massive brain swelling and/or obliteration of cistern structures on computed tomography scan because of increased ICP. DC surgery was performed as an initial treatment option in ICH patients and it as an additional treatment after medical treatment in MI patients. Patients manifesting other systemic disease (such as malignancy, severe heart problems, etc.), GCS score of 3, no self-respiration, and family refusing aggressive treatment, were excluded from this study42). After DC surgery, ICP targeted conventional medical management included hyperosmotic agents, and if ventricular pressure exceeded 15 mmHg, opened the extraventricular drainage (EVD). And if the ICP was over 25 mmHg, tied metabolic therapy, or reoperation for ICH, or opposite side DC were attempted.
In all patients, operations were carried out under general endotracheal anesthesia and in the supine position. The ventricular puncture was routinely performed at Kocher’s point on the right side but left side ventricle was punctured when the hematoma or massive brain edema placed on the right side, if it make difficult to right side ventricular puncture. The EVD tube (EVD catheter; Yushin Medical, Seoul, Korea) was connected to the continuous monitor (CPP-monitor; Spiegelberg, Munich, Germany) via a transducer device (Druckmeß-set; Smiths industries, Munchen, Germany). A large bicoronal or unilateral skin flap was made according to the underlying pathologic brain condition. After the initial ventricular ICP was stabilized, the burr holes were connected with a pneumatic saw and the bone flap with a diameter of at least 15×12 cm was subsequently removed. Additional bone was removed at the temporal region to the floor of the middle fossa (Fig. 2) as far as the surgical position permitted. After the craniectomy was completed, the dura was opened in a large cruciated or curved Z-shaped incision, in the areas involving the frontal, temporal and parietal lobes. In all patients, artificial dura was placed underneath the incised dura, securing it with several sutures, to allow the brain to herniate outward in a more controlled manner. And thin surgicel pieces were placed between the skin flap and dura to prevent cortical adhesion and it make easy to dissection for later cranioplasty. The temporalis muscle and skin flap were then reapproximated with sutures8,23,38,42). Typically, the bone flap was maintained in wet gauze at −70°C until reinsertion, which occurred 1–3 months after the initial surgery. This study was not a randomly controlled trial and the decision to apply unilateral or bilateral DC was determined by the operating surgeon.
Data collected included age, sex, initial GCS, initial ICP, postoperative ventricular ICP, and clinical results. Initial ICP was defined as the intracranial pressure immediately following ventricular puncture. Postoperative ICP data were collected every two hours for at least 3 days after the surgery (range, 3–10 days).
Neurologic outcomes were evaluated at 6 months after the initial treatment by a neurosurgeon who was not involved in the patients’ initial management. Neurologic outcomes were quantified using the extended Glasgow outcome scale (GOS) (favorable outcome : Glacow outcome scale extented [GOSE] 5–8; unfavorable outcome : GOSE 2–4 and mortality rate).
All data are presented as the mean±standard deviation and/or the median. A Wilcoxon signed-rank sum test was used to analyze GCS and GOSE. Comparisons among CPP grading values were computed using the unpaired t-test and Fisher exact test. Statistical analyses for each outcome were analyzed with SPSS software version 12 (SPSS Inc., Chicago, IL, USA). For all statistical analyses, significance was defined by a p≤0.05.
The initial clinical features were similar among both groups (Table 1). Age (years) was 57.2±13.3 years (mean=56.0) in MI patients and 55.6±14.9 years (mean=57.0) in ICH patients (p=0.652). Initial GCS was 6.7±1.7 in MI patients and 6.4±2.0 in ICH patients (p=0.364). Initial ventricular ICP was 32.3±18.2 mmHg (mean=27.4) in MI patients and 45.2±18.7 mmHg (median=42.0) in ICH patients (p=0.783).
The OP-time, time lapse from ictus to DC was significantly different among the two groups (40.9±44.9 hours; median=31.5, MI patients vs. 7.3±3.4 hours; median=6.0, ICH patients, p=0.000).
The postoperative ICP values of ICH patients were maintained within the physiological range if greater than 80% of the hematoma was removed. In MI patients, however, the postoperative ICP values were higher on average (18.8 mmHg, MI patients vs. 13.6 mmHg, ICH patients, p=0.000) and more irregular than ICH patients (Fig. 2).
The mortality rate was higher in MI patients (MI patients=37.1% vs. ICH patients=17.3%, p=0.007) than ICH patients, but the likelihood of favorable outcomes was not significant different among the two groups (p=0.163).
Ninety-seven patients underwent unilateral DC and 46 patients underwent bilateral DC (Fig. 3). Initial neurologic status evaluated by GCS was similar among the groups (GCS=6.6 in unilateral DC vs. GCS=6.3 in bilateral DC, p=0.810). And when clinical outcomes were compared, the likelihood of a favorable outcome (χ2, p=0.207) was not significantly different among the two groups. Mortality, however was significantly higher in bilateral vs. unilateral DC patients (χ2, p=0.000).
Many authors have reported on the benefits and limitations of DC in patients with various neurosurgical diseases, such as ICH11,29,38,40), SAH5,6,14,30,33,35,36), MI1,16,19,21,23,34,39), and severe TBI4,8,10,17,23,36). In authors’ institutes DC performed on various neurosurgical diseases for the management of increased ICP22,42).
DC involves removal of the large areas of the calvarium, and converting the intracranial space from a ‘closed system’ with a finite volume into an ‘open system,’ thereby shift to the right of the pressure-volume curve and improve the brain physiology4,9,18,22–25,34,36,38,42). These pathophysiological processes result in effective lowering of increased ICP, improvement in cerebral perfusion and prevention of secondary brain damages4,9,10,13,15,18,23,34,36,38,41). Some reports indicate that DC might be more effective in MI than TBI patients, because the biomechanics of TBI may be more diffuse than that of MI. But according to the author’s previous report, DC surgery was found to be more effective in patients with ICH and TBI than MI22).
Massive ICH, MI and SAH are devastating cerebral vascular accident associated with high mortality and morbidity, despite optimum management2,3,16,37). In this analysis, postoperative ICP trend of the ruptured SAH was not include. Because some SAH patients developed vasospasm 5–7 days after the initial operation, the ICP trend hardly saved for the limitation of EVD maintenance duration. And it was difficult to evaluate the clinical outcome, because it was influenced mainly not by ICP rather by delayed neurologic deficit caused from vasospasm.
In massive ICH patients, the postoperative ICP dropped rapidly and maintained within the physiological range after DC surgery if the hematoma was removed more than 80%. However, in patients with postoperative ICP exceeding 25 mmHg, an insignificant amount of the hematoma was removed (less than 80%), increased hematoma amount by rebleeding or massive brain swelling caused by hematoma contents. In 7 ICH patients, hematoma was not removed enough (less than 80%) required second operation to remove the remained hematoma because of high postoperative ICP trend.
Despite the improved control of systemic hypertension, massive ICH is still one of the most devastating forms of cerebrovascular disease5,27,29,44). According to the previous clinical and animal study reports, that ICP increases for 3 days again after a few hours after removal of the hematoma because the hemorrhage itself triggers a series of negative pathogenic mechanisms and brain edema, the final result of which is the loss of cerebral autoregulation and development of edema7,9,12,20,27,28). Besides hematoma volume, perihemorrhagic edema may cause secondary deterioration of ICH patients32,43). Driven by recent promising results of DC in ischemic stroke, DC could also be promising for treatment of space occupying ICH12,19,39). Our results show that in ICH patient treated with DC surgery, postoperative ICP trend remain stable and within the physiologic range with conventional additional medical management if the hematoma removed greater than 80%.
While in MI patients, the postoperative ICP did not dropped as rapidly as the ICH patients and usually it was maintained above the physiologic level for several days after DC. Postoperative ICP trend in MI patients was a good indicator for the additional treatment application, such as hyperosmotic diuretics, cerebrospinal fluid drainage, or metabolic therapy.
According to previous papers, MI is accompanied by a vicious cycle where ischemic insult leads to further edema, and thus to increases the ICP and reduction of regional cerebral blood flow26,34). Patients with malignant MCA infarction in who DC was undertook within 48 hours of stroke onset, experience reductions in mortality and an increase in the likelihood of a favorable functional outcome3,16,26,38). ICP should be monitored in patients undergoing DC to better understand the ongoing risk of cerebral edema, herniation and timely application of additional treatment.
There is a possibility that surgical decompression raises the risk of rebleeding, particularly in DC patients showed increasing hematoma following surgery12). In our study, some patients showed hematoma regrowth in ICH patients and hemorrhagic transformation in MI patients but the risk of hematoma enlargement or formation were within the natural course12).
Until now, there were no reports comparing the effectiveness between unilateral and bilateral DC. Our study was not intended to compare the effectiveness of unilateral or bilateral DC. And the unilateral or bilateral DC was decided by the operating surgeon, not by randomized method. Unilateral DC was applied in 97 cases, while bilateral DC was applied in 46 cases. The initial GCS was not different between the unilateral and bilateral DC patients, but the clinical outcomes were better in unilateral DC patients. We can’t conclude the superiority of the unilateral or bilateral DC surgical methods. But we would like to hypothesize that massive ICH or MI, most significant ICP might be developed at the basal ganglia and tempo-parietal lobe, that cause increased ICP was focused on one side (not diffuse), so wide decompression on the pathologic site might be more important than total area of decompression.
There are several limitation to this study, it was a retrospective, observational study with a small number of patients and did not include a control group, precluding generalization of these findings to all patients who undergo DC following ICH or MI. On this paper, author can’t prove the significance of hematoma removal more than 80%, because of the case limitation of this study (reoperation cases was 6 patients) and ICH patients with small amount (about 30–80 mL hematoma volume) undertook hematoma puncture with catheter or hematoma remove operation without monitoring. Nevertheless, we suspect that this study can valuably contribute to discussions regarding the clinical significance of postoperative ICP monitoring in patients with massive ICH and MI.
According to our findings, if a significant enough portion of the hematoma is removed from patients experience ICH, craniotomy can be applied rather than craniectomy. Additionally, if the postoperative ICP increases again after a stable period, additional medical treatment and/or additional DC surgery should be considered. From this study, authors suggest that in increased ICP patients with cerebral vascular disease, wide unilateral craniectomy with sufficient temporal base decompression is more important than total area of decompression.
From this study, the observed trends in postoperative ICP were different between ICH and MI patients. In massive ICH patients, the postoperative ICP values dropped rapidly and were maintained within physiologic levels if a significant enough portion (more than 80%) of the hematoma was removed during DC. In such ICH cases, authors recommend craniotomy rather than craniectomy. While in MI patients, postoperative ICP values did not drop as rapidly as ICH patients and in most cases required additional treatment. Postoperative ICP monitoring was useful to decision making for the additional treatment.
Acknowledgements
This research was supported by a grant from Catholic Institute of Cell Therapy in 2016, and Basic Research Fund form Yohan Pharmacy 2016.
References
1. Al-Jehani H, Petrecca K, Martel P, Sinclair D, Sirhan D. Decompressive craniectomy for ischemic stroke: effect of hemorrhagic transformation on outcome. J Stroke Cerebrovasc Dis. 25:2177–2183. 2016.

2. Anderson CS, Chakera TM, Stewart-Wynne EG, Jamrozik KD. Spectrum of primary intracerebral haemorrhage in Perth, Western Australia, 1989–90: incidence and outcome. J Neurol Neurosurg Psychiatry. 57:936–940. 1994.

3. Arac A, Blanchard V, Lee M, Steinberg GK. Assessment of outcome following decompressive craniectomy for malignant middle cerebral artery infarction in patients older than 60 years of age. Neurosurg Focus. 26:E3. 2009.

4. Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons; Joint Section on Neurotrauma and Critical Care, AANS/CNS. Bratton SL, Chestnut RM, et al. Guidelines for the management of severe traumatic brain injury. VIII Intracranial pressure thresholds. J Neurotrauma. 24:Suppl 1. S55–S58. 2007.
5. Broderick JP, Adams HP Jr, Barsan W, Feinberg W, Feldmann E, Grotta J, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a statement for healthcare professionals from a special writing group of the stroke council, American Heart Association. Stroke. 30:905–915. 1999.

6. Broderick JP, Brott T, Tomsick T, Miller R, Huster G. Intracerebral hemorrhage more than twice as common as subarachnoid hemorrhage. J Neurosurg. 78:188–191. 1993.

7. Brott T, Broderick J, Kothari R, Barsan W, Tomsick T, Sauerbeck L, et al. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. 28:1–5. 1997.

8. Carney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GW, Bell MJ, et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. 80:6–15. 2017.

9. Cooper PR, Rovit RL, Ransohoff J. Hemicraniectomy in the treatment of acute subdural hematoma: a re-appraisal. Surg Neurol. 5:25–28. 1976.
10. Csókay A, Pataki G, Nagy L, Belán K. Vascular tunnel construction in the treatment of severe brain swelling caused by trauma and SAH. (evidence based on intra-operative blood flow measure). Neurol Res. 24:157–160. 2002.

11. Dierssen G, Carda R, Coca JM. The influence of large decompressive craniectomy on the outcome of surgical treatment in spontaneous intracerebral haematomas. Acta Neurochir (Wien). 69:53–60. 1983.

12. Fung C, Murek M, Z’Graggen WJ, Krähenbühl AK, Gautschi OP, Schucht P, et al. Decompressive hemicraniectomy in patients with supratentorial intracerebral hemorrhage. Stroke. 43:3207–3211. 2012.

13. Gaab MR, Rittierodt M, Lorenz M, Heissler HE. Traumatic brain swelling and operative decompression: a prospective investigation. Acta Neurochir Suppl (Wien). 51:326–328. 1990.

14. Güresir E, Schuss P, Vatter H, Raabe A, Seifert V, Beck J. Decompressive craniectomy in subarachnoid hemorrhage. Neurosurg Focus. 26:E4. 2009.

15. Hatashita S, Hoff JT. The effect of craniectomy on the biomechanics of normal brain. J Neurosurg. 67:573–548. 1987.

16. Hofmeijer J, Kappelle LJ, Algra A, Amelink GJ, van Gijn J, van der Worp HB. Surgical decompression for space-occupying cerebral infarction (the hemicraniectomy after middle cerebral artery infarction with life-threatening edema trial [HAMLET]): a multicentre, open, randomised trial. Lancet Neurol. 8:326–333. 2009.

17. Hutchinson PJ, Kolias AG, Timofeev IS, Corteen EA, Czosnyka M, Timothy J, et al. Trial of decompressive craniectomy for traumatic intracranial hypertension. N Engl J Med. 375:1119–1130. 2016.

18. Jaeger M, Soehle M, Meixensberger J. Effects of decompressive craniectomy on brain tissue oxygen in patients with intracranial hypertension. J Neurol Neurosurg Psychiatry. 74:513–515. 2003.

19. Jüttler E, Schwab S, Schmiedek P, Unterberg A, Hennerici M, Woitzik J, et al. Decompressive surgery for the treatment of malignant infarction of the middle cerebral artery (DESTINY): a randomized, controlled trial. Stroke. 38:2518–2525. 2007.

20. Kazui S, Naritomi H, Yamamoto H, Sawada T, Yamaguchi T. Enlargement of spontaneous intracerebral hemorrhage. Incidence and time course Stroke. 27:1783–1787. 1996.

21. Kenning TJ, Gooch MR, Gandhi RH, Shaikh MP, Boulos AS, German JW. Cranial decompression for the treatment of malignant intracranial hypertension after ischemic cerebral infarction: decompressive craniectomy and hinge craniotomy. J Neurosurg. 116:1289–1298. 2012.

22. Kim KT, Park JK, Kang SG, Cho KS, Yoo DS, Jang DK, et al. Comparison of the effect of decompressive craniectomy on different neurosurgical diseases. Acta Neurochir (Wien). 151:21–30. 2009.

23. Kurzbuch AR. Does size matter? Decompressive surgery under review. Neurosurg Rev. 38:629–640. 2015.

24. Macdonell RAL, Kalnins RM, Donnan GA. Cerebellar infarction: natural history, prognosis, and pathology. Stroke. 18:849–855. 1987.

25. Maira G, Anile C, Colosimo C, Rossi GF. Surgical treatment of primary supratentorial intracerebral hemorrhage in stuporous and comatose patients. Neurol Res. 24:54–60. 2002.

26. Maramattom BV, Bahn MM, Wijdicks EF. Which patient fares worse after early deterioration due to swelling from hemispheric stroke? Neurology. 63:2142–2145. 2004.

27. Marinkovic I, Strbian D, Pedrono E, Vekovischeva OY, Shekhar S, Durukan A, et al. Decompressive craniectomy for intracerebral hemorrhage. Neurosurgery. 65:780–786. 2009.

28. Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, et al. Recombinant activated factor VII intracerebral hemorrhage Trial Investigators: Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 352:777–785. 2005.

29. Mitchell P, Gregson BA, Vindlacheruvu RR, Mendelow AD. Surgical options in ICH including decompressive craniectomy. J Neurol Sci. 261:89–98. 2007.

30. Otani N, Takasato Y, Masaoka H, Hayakawa T, Yoshino Y, Yatsushige H, et al. Surgical outcome following decompressive craniectomy for poor-grade aneurysmal subarachnoid hemorrhage in patients with associated massive intracerebral or sylvian hematomas. Cerebrovasc Dis. 26:612–617. 2008.

31. Paldor I, Rosenthal G, Cohen JE, Leker R, Harnof S, Shoshan Y, et al. Intracranial pressure monitoring following decompressive hemicraniectomy for malignant cerebral infarction. J Clin Neurosci. 22:79–82. 2015.

32. Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 377:31–41. 2011.

33. Schirmer CM, Hoit DA, Malek AM. Decompressive hemicraniectomy for the treatment of intractable intracranial hypertension after aneurysmal subarachnoid hemorrhage. Stroke. 38:987–992. 2007.

34. Slotty PJ, Kamp MA, Beez T, Beenen H, Steiger HJ, Turowski B, et al. The influence of decompressive craniectomy for major stroke on early cerebral perfusion. J Neurosurg. 123:59–64. 2015.

35. Smith ER, Carter BS, Ogilvy CS. Proposed use of prophylactic decompressive craniectomy in poor-grade aneurysmal subarachnoid hemorrhage patients presenting with associated large sylvian hematomas. Neurosurgery. 51:117–124. 2002.

36. Strege RJ, Lang EW, Stark AM, Scheffner H, Fritsch MJ, Barth H, et al. Cerebral edema leading to decompressive craniectomy: an assessment of the preceding clinical and neuromonitoring trends. Neurol Res. 25:510–515. 2003.

37. Toni D, Fiorelli M, Gentile M, Bastianello S, Sacchetti ML, Argentino C, et al. Progressing neurological deficit secondary to acute ischemic stroke. A study on predictability, pathogenesis, and prognosis. Arch Neurol. 52:670–675. 1995.

38. Tagliaferri F, Zani G, Iaccarino C, Ferro S, Ridolfi L, Basaglia N, et al. Decompressive craniectomies, facts and fiction: a retrospective analysis of 526 cases. Acta Neurochir (Wien). 154:919–926. 2012.

39. Vahedi K, Vicaut E, Mateo J, Kurtz A, Orabi M, Guichard JP, et al. Sequential-design, multicenter, randomized, controlled trial of early decompressive craniectomy in malignant middle cerebral artery infarction (DECIMAL Trial). Stroke. 38:2506–2517. 2007.

40. Xiao B, Wu FF, Zhang H, Ma YB. A randomized study of urgent computed tomography-based hematoma puncture and aspiration in the emergency department and subsequent evacuation using craniectomy versus craniectomy only. J Neurosurg. 117:566–573. 2012.

41. Yamakami I, Yamaura A. effects of decompressive craniectomy on regional cerebral blood flow in severe head trauma patients. Neruol Med Chir (Tokyo). 33:616–620. 1993.

42. Yoo DS, Kim DS, Cho KS, Huh PW, Park CK, Kang JK. Ventricular pressure monitoring during bilateral decompression with dural expansion. J Neurosurg. 91:953–959. 1999.

Fig. 1
Treatment protocol outline. GCS : Glasgow coma scale, IICP : increased intracranaila pressure, CT : computed tomography, ICP : intracranaila pressure, CSF : cerebrospinal fluid, Tx : treatment.
Fig. 2
Postoperative intracranaial pressure trends. A : Intracerebral hemorrhage. B : Major infarction. Red line : expired patients, Dotted line : reoperation cases.
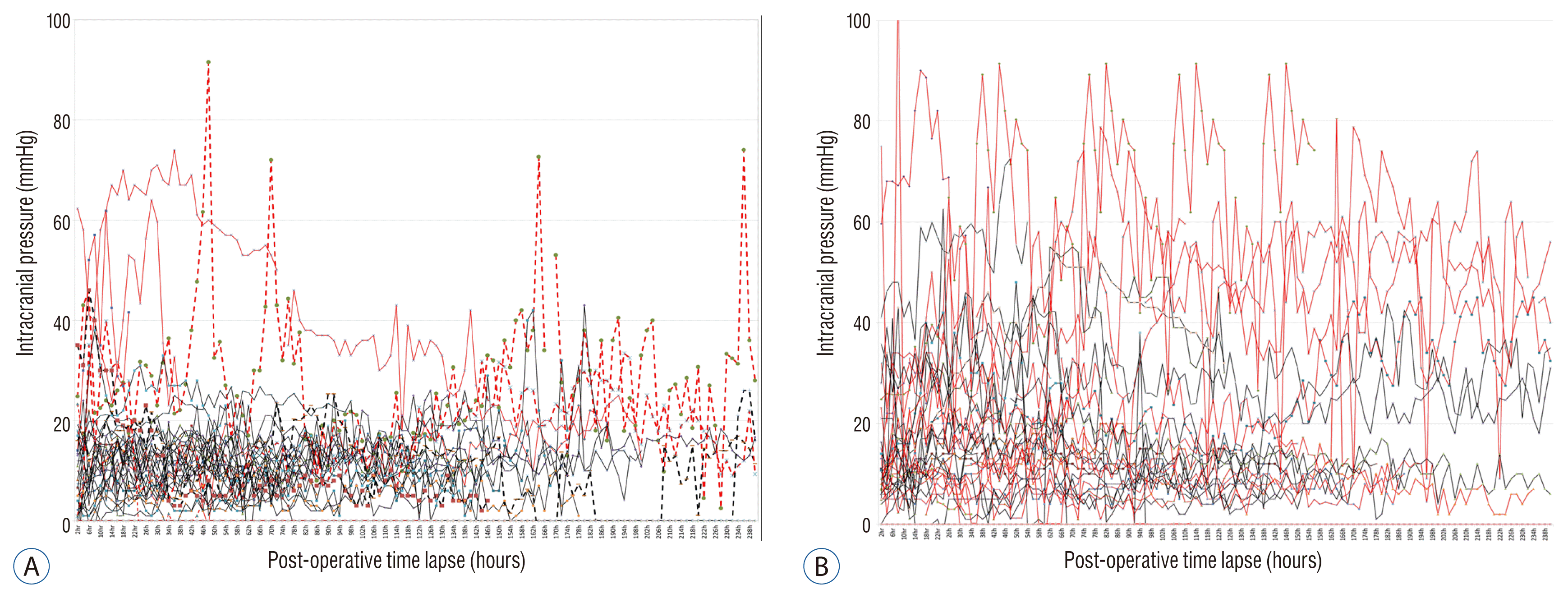
Fig. 3
3-dimensional brain computed tomography after craniectomy. A : Bilateral decompression. B : Unilateral decompression.
Table 1
Patients clinical features and clinical outcomes




 Citation
Citation Print
Print


 XML Download
XML Download